EX-99.2
Published on August 21, 2023

Transaction & Overview Q3 2023 +

skyebioscience.com • 2 FORWARD LOOKING STATEMENTS This presentation contains “forward-looking statements”, including statements regarding Skye Bioscience, Inc. (“Skye Bioscience and/or the Company”), within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. All statements in this presentation, whether written or oral, that are not descriptions of historical facts or that refer to expected or anticipated future actions and results of Skye Bioscience are forward-looking statements, including statements regarding Skye Bioscience’s cash runway, anticipated timelines, and milestones with respect to product development programs, business strategy, expected plans with respect to clinical trials, including the timing of patient enrollment and clinical trial data updates, and commercialization, if ever, of endocannabinoid system-targeting and CB1-targeting therapeutics. In addition, any statements that refer to expectations, projections or other characterizations of future events or circumstances are forward-looking statements. Forward-looking statements can be identified by terminology including “anticipated,” “plans,” “goal,” “focus,” “aims,” “intends,” “believes,” “can,” “could,” “challenge,” “predictable,” “will,” “would,” “may” or the negative of these terms or other comparable terminology. Forward-looking statements reflect our current projections and expectations about future events as of the date of this presentation and involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements, including those uncertainties and factors described in Skye’ Bioscience’s most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission and our subsequent periodic reports filed with the Securities and Exchange Commission. Skye Bioscience cannot give any assurance that such forward-looking statements will prove to be correct. The reader is cautioned not to place undue reliance on these forward-looking statements. All information contained in this presentation is provided as of the date of the presentation and is subject to change without notice. The information contained in this presentation does not purport to contain all the information that may be necessary or desirable to fully and accurately evaluate an investment in Skye Bioscience. Neither Skye Bioscience, nor any other person, undertakes any obligation to update or revise publicly any of the forward-looking statements set out herein, whether as a result of new information, future events or otherwise, except as required by law. This presentation shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities of Skye Bioscience, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. This is presented as a source of information and not an investment recommendation.

TRANSACTION HIGHLIGHTS $17M institutionally-led financing • $12M PIPE led by 5AM Ventures; joined by Versant Ventures and other accredited investors • $5M convertible note Bird Rock Bio acquisition • $20M merger consideration for Bird Rock’s outstanding equity interests held by dedicated biotechnology investors exchanged for Skye common stock Management and board of directors • Existing Skye management team will continue to lead the company • Existing board of directors expanded with addition of Andy Schwab (Managing Partner, 5AM Ventures) and Paul Grayson (Venture Partner, Versant & CEO, Tentarix) Primary intended use of proceeds • SBI-100 OE glaucoma: prepare/report Phase 1 data for SBI-100 OE in Q3; start Phase 2a enrollment in Q4; interim Phase 2a data 1H ’24 • Develop nimacimab Phase 2 clinical strategy for chronic kidney disease. Initiate Phase 2a H1 2024 • Expected cash runway into 2024. skyebioscience.com • 3 +
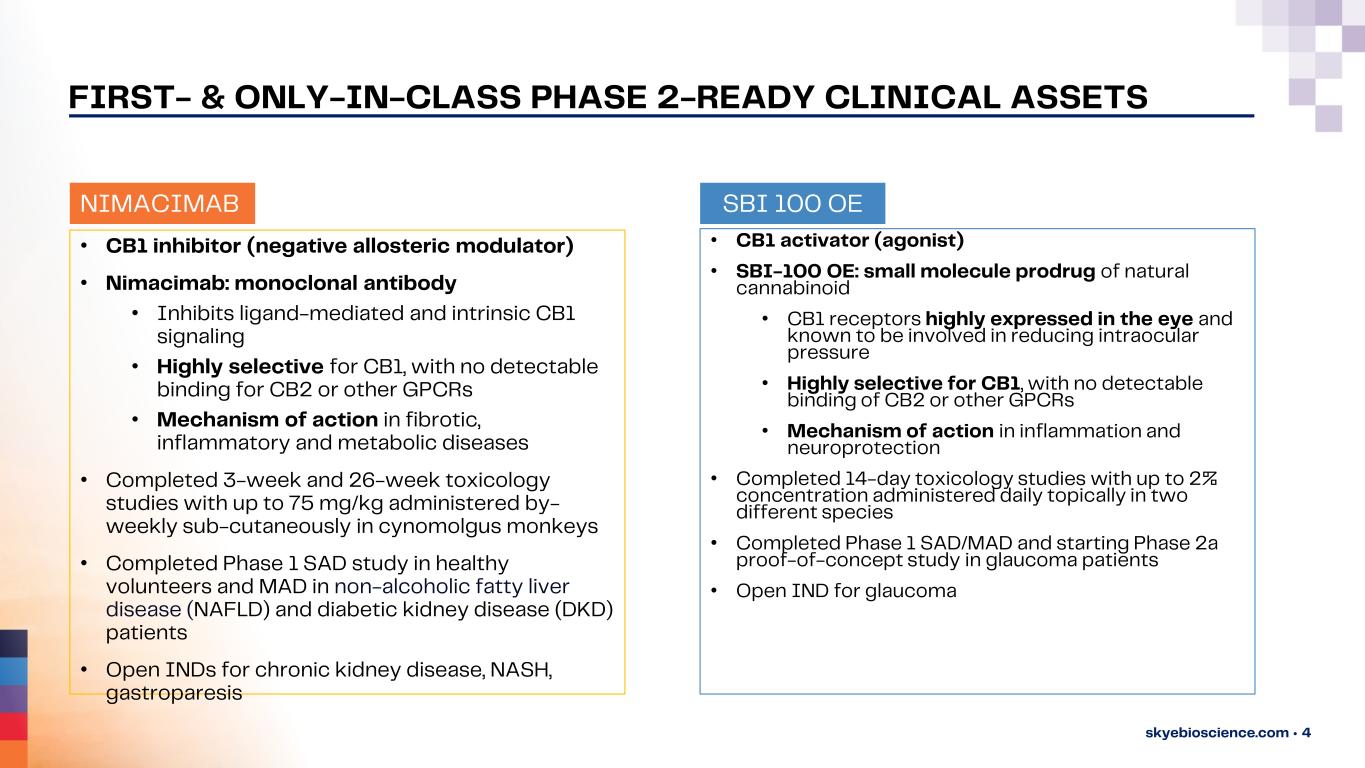
skyebioscience.com • 4 • CB1 inhibitor (negative allosteric modulator) • Nimacimab: monoclonal antibody • Inhibits ligand-mediated and intrinsic CB1 signaling • Highly selective for CB1, with no detectable binding for CB2 or other GPCRs • Mechanism of action in fibrotic, inflammatory and metabolic diseases • Completed 3-week and 26-week toxicology studies with up to 75 mg/kg administered by- weekly sub-cutaneously in cynomolgus monkeys • Completed Phase 1 SAD study in healthy volunteers and MAD in non-alcoholic fatty liver disease (NAFLD) and diabetic kidney disease (DKD) patients • Open INDs for chronic kidney disease, NASH, gastroparesis • CB1 activator (agonist) • SBI-100 OE: small molecule prodrug of natural cannabinoid • CB1 receptors highly expressed in the eye and known to be involved in reducing intraocular pressure • Highly selective for CB1, with no detectable binding of CB2 or other GPCRs • Mechanism of action in inflammation and neuroprotection • Completed 14-day toxicology studies with up to 2% concentration administered daily topically in two different species • Completed Phase 1 SAD/MAD and starting Phase 2a proof-of-concept study in glaucoma patients • Open IND for glaucoma SBI 100 OENIMACIMAB FIRST- & ONLY-IN-CLASS PHASE 2-READY CLINICAL ASSETS
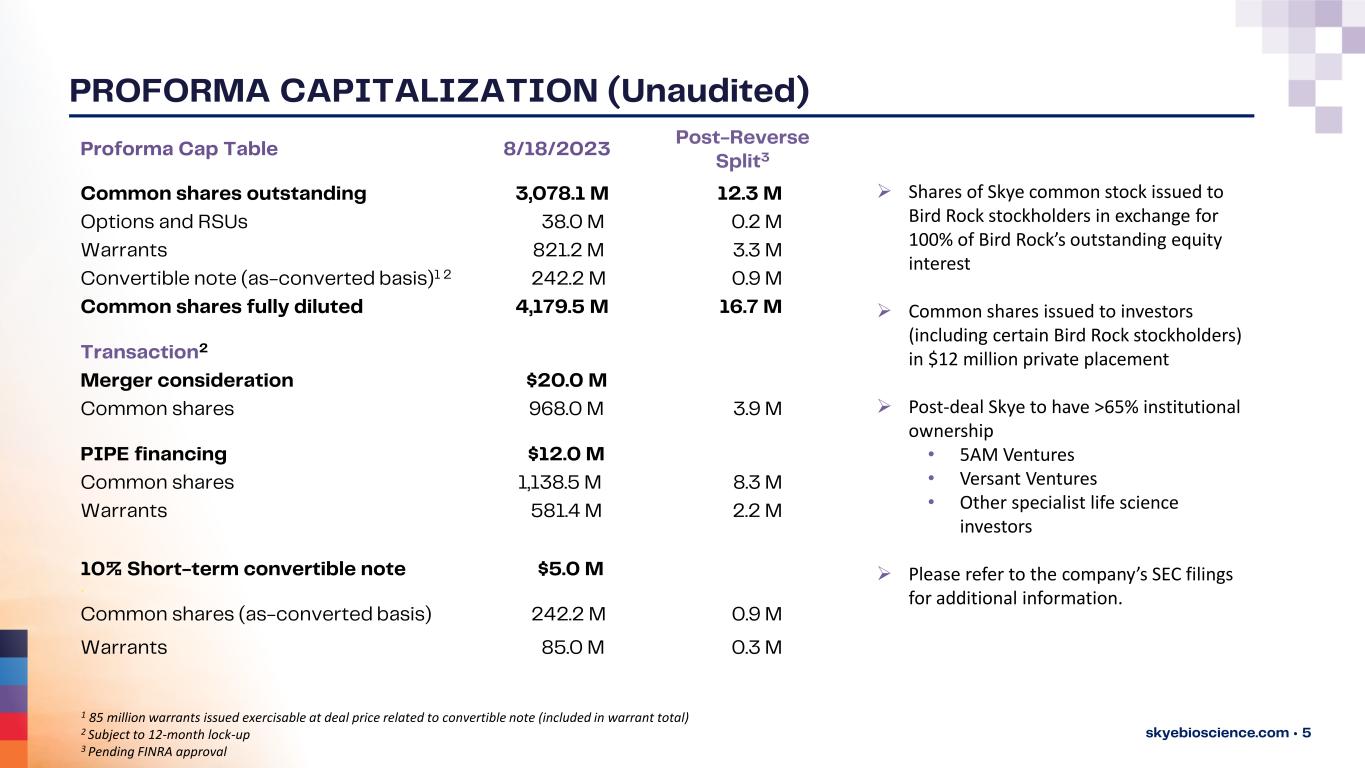
PROFORMA CAPITALIZATION (Unaudited) skyebioscience.com • 5 Proforma Cap Table 8/18/2023 Common shares outstanding 3,078.1 M 12.3 M Options and RSUs 38.0 M 0.2 M Warrants 821.2 M 3.3 M Convertible note (as-converted basis)1 2 242.2 M 0.9 M Common shares fully diluted 4,179.5 M 16.7 M Transaction2 Merger consideration $20.0 M Common shares 968.0 M 3.9 M PIPE financing $12.0 M Common shares 1,138.5 M 8.3 M Warrants 581.4 M 2.2 M 10% Short-term convertible note . $5.0 M Common shares (as-converted basis) 242.2 M 0.9 M Warrants 85.0 M 0.3 M ➢ Shares of Skye common stock issued to Bird Rock stockholders in exchange for 100% of Bird Rock’s outstanding equity interest ➢ Common shares issued to investors (including certain Bird Rock stockholders) in $12 million private placement ➢ Post-deal Skye to have >65% institutional ownership • 5AM Ventures • Versant Ventures • Other specialist life science investors ➢ Please refer to the company’s SEC filings for additional information. 1 85 million warrants issued exercisable at deal price related to convertible note (included in warrant total) 2 Subject to 12-month lock-up 3 Pending FINRA approval Post-Reverse Split3
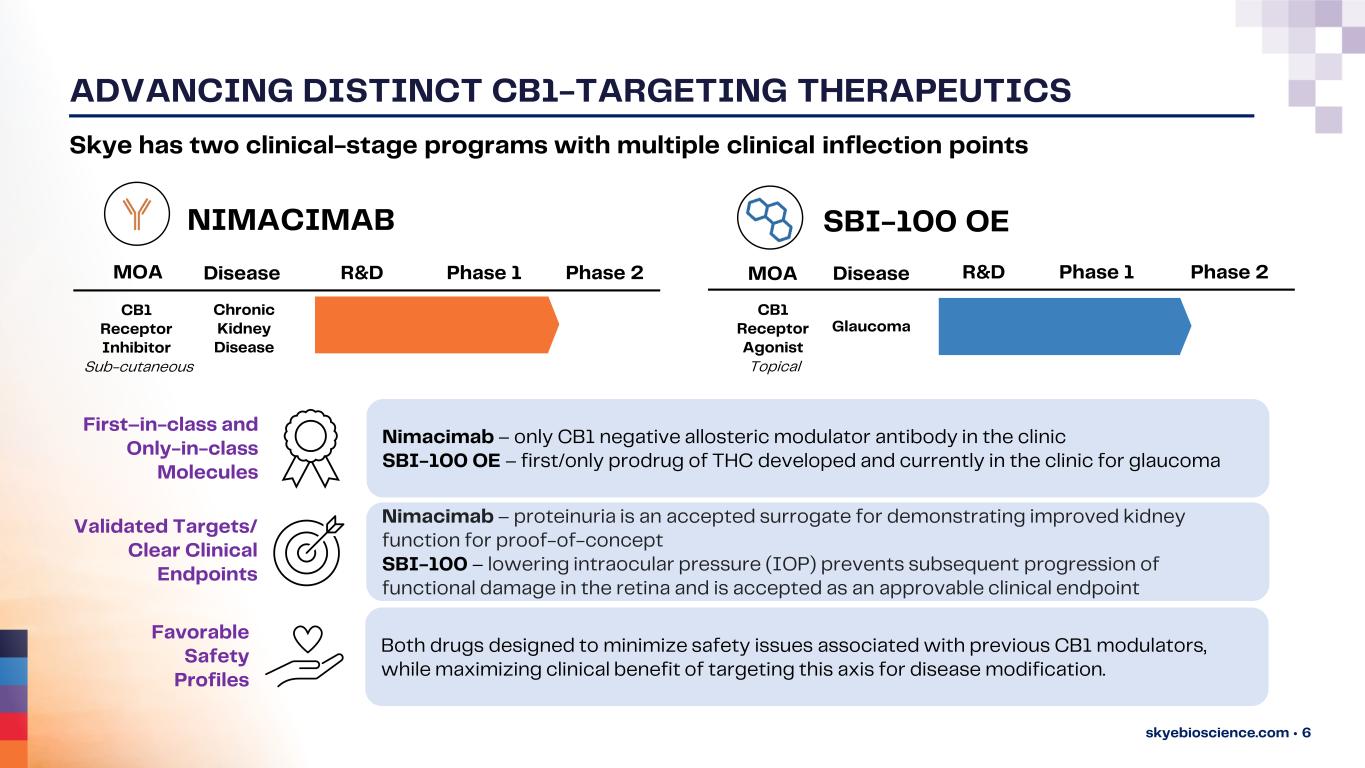
ADVANCING DISTINCT CB1-TARGETING THERAPEUTICS skyebioscience.com • 6 R&D Phase 1 Phase 2MOA CB1 Receptor Inhibitor Chronic Kidney Disease Disease R&D Phase 1 Phase 2MOA CB1 Receptor Agonist Glaucoma Disease Skye has two clinical-stage programs with multiple clinical inflection points First–in-class and Only-in-class Molecules Validated Targets/ Clear Clinical Endpoints Favorable Safety Profiles SBI-100 OENIMACIMAB Nimacimab – only CB1 negative allosteric modulator antibody in the clinic SBI-100 OE – first/only prodrug of THC developed and currently in the clinic for glaucoma Nimacimab – proteinuria is an accepted surrogate for demonstrating improved kidney function for proof-of-concept SBI-100 – lowering intraocular pressure (IOP) prevents subsequent progression of functional damage in the retina and is accepted as an approvable clinical endpoint Both drugs designed to minimize safety issues associated with previous CB1 modulators, while maximizing clinical benefit of targeting this axis for disease modification. TopicalSub-cutaneous
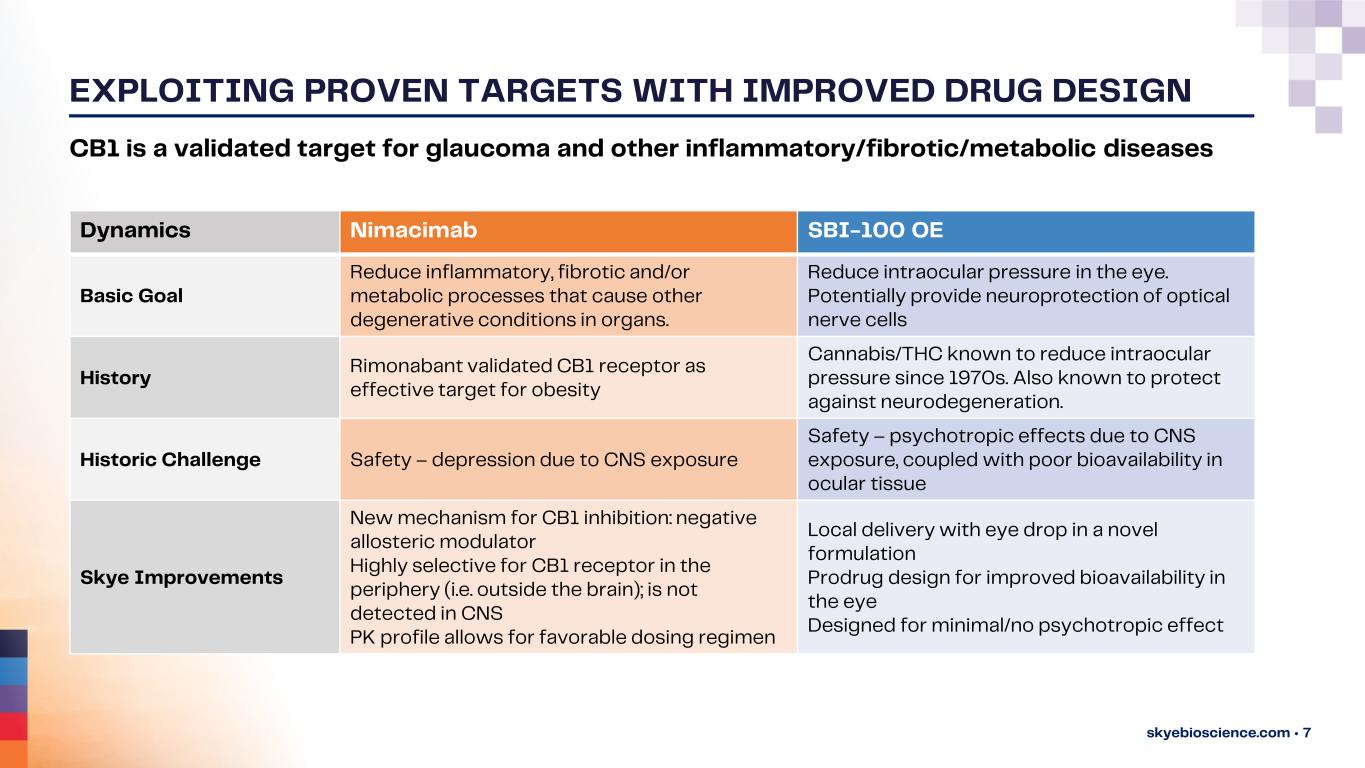
EXPLOITING PROVEN TARGETS WITH IMPROVED DRUG DESIGN skyebioscience.com • 7 CB1 is a validated target for glaucoma and other inflammatory/fibrotic/metabolic diseases Dynamics Nimacimab SBI-100 OE Basic Goal Reduce inflammatory, fibrotic and/or metabolic processes that cause other degenerative conditions in organs. Reduce intraocular pressure in the eye. Potentially provide neuroprotection of optical nerve cells History Rimonabant validated CB1 receptor as effective target for obesity Cannabis/THC known to reduce intraocular pressure since 1970s. Also known to protect against neurodegeneration. Historic Challenge Safety – depression due to CNS exposure Safety – psychotropic effects due to CNS exposure, coupled with poor bioavailability in ocular tissue Skye Improvements New mechanism for CB1 inhibition: negative allosteric modulator Highly selective for CB1 receptor in the periphery (i.e. outside the brain); is not detected in CNS PK profile allows for favorable dosing regimen Local delivery with eye drop in a novel formulation Prodrug design for improved bioavailability in the eye Designed for minimal/no psychotropic effect
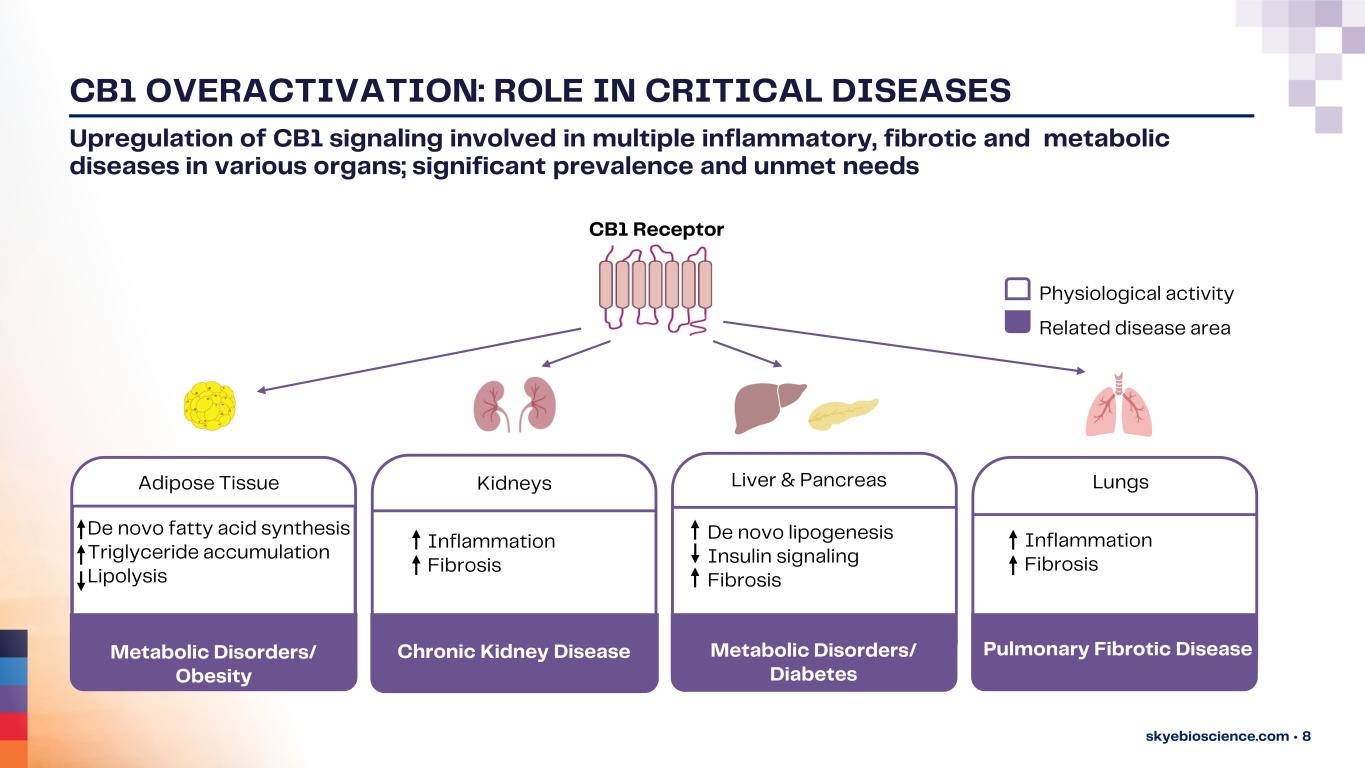
CB1 OVERACTIVATION: ROLE IN CRITICAL DISEASES skyebioscience.com • 8 Upregulation of CB1 signaling involved in multiple inflammatory, fibrotic and metabolic diseases in various organs; significant prevalence and unmet needs Adipose Tissue Kidneys LungsLiver & Pancreas CB1 Receptor Metabolic Disorders/ Obesity De novo fatty acid synthesis Triglyceride accumulation Lipolysis De novo lipogenesis Insulin signaling Fibrosis Inflammation Fibrosis Chronic Kidney Disease Fibrotic Liver Disease <pancreas-related?> Physiological activity Related disease area Pulmonary Fibrotic DiseaseMetabolic Disorders/ Diabetes Inflammation Fibrosis
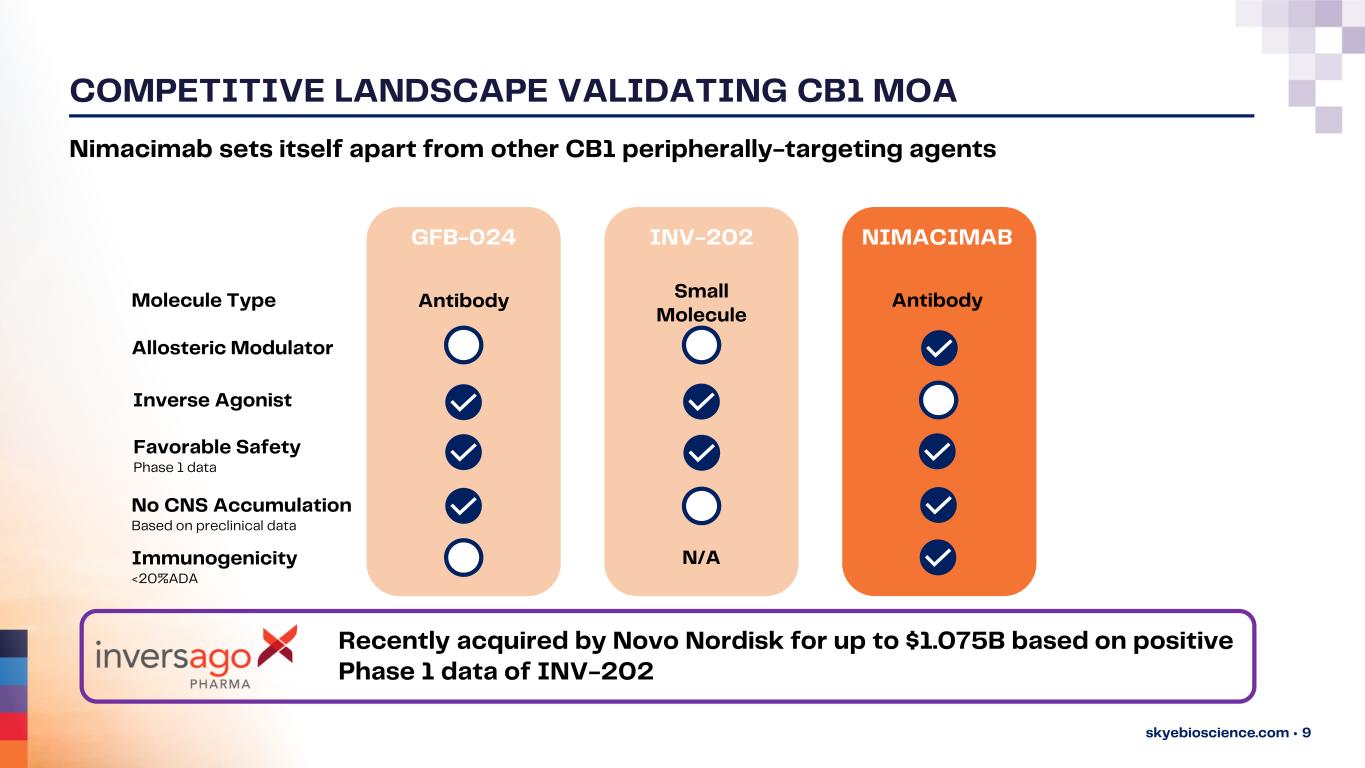
COMPETITIVE LANDSCAPE VALIDATING CB1 MOA skyebioscience.com • 9 Recently acquired by Novo Nordisk for up to $1.075B based on positive Phase 1 data of INV-202 NIMACIMABINV-202GFB-024 Molecule Type No CNS Accumulation Based on preclinical data Allosteric Modulator Inverse Agonist Favorable Safety Phase 1 data Immunogenicity <20%ADA Antibody Antibody Small Molecule N/A Nimacimab sets itself apart from other CB1 peripherally-targeting agents
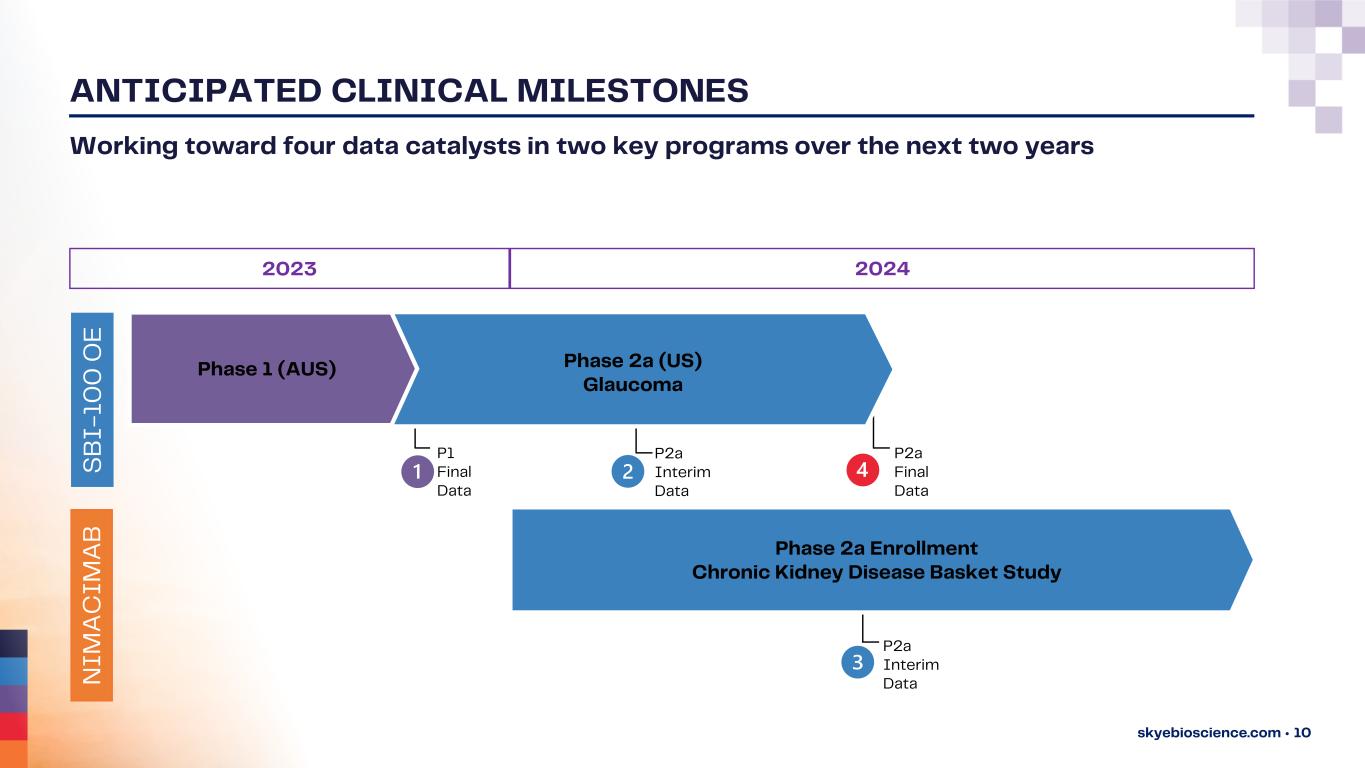
ANTICIPATED CLINICAL MILESTONES skyebioscience.com • 10 Working toward four data catalysts in two key programs over the next two years P2a Interim Data Phase 2a Enrollment Chronic Kidney Disease Basket Study Phase 1 (AUS) Phase 2a (US) Glaucoma 2023 2024 P1 Final Data P2a Interim Data P2a Final Data N I M A C I M A B S B I - 1 0 0 O E

skyebioscience.com • 11 LEADERSHIP Tu Diep, MSc Chief Development Officer Chris Twitty, PhD Chief Scientific Officer Kaitlyn Arsenault, CPA Chief Financial Officer Punit Dhillon CEO & Chair of BOD Margaret Dalesandro, PhD Pharma. Dev. Consultant, Brecon Pharma Consulting Deborah Charych, PhD Co-founder and former CTO, RayzeBio Keith Ward, PhD Founder, Pres./CEO, & Chair, Kuria Therapeutics E x e c u ti v e M a n a g e m e n t B o a rd o f D ir e c to rs Andy Schwab Managing Partner, 5AM Ventures Paul Grayson Pres./CEO, Tentarix Bio; Versant partner Praveen Tyle, PhD Founder, Potens Pharma Contributed to commercialization of 47+ drugs/diagnostics, led high-value strategic transactions and co-founded multiple companies
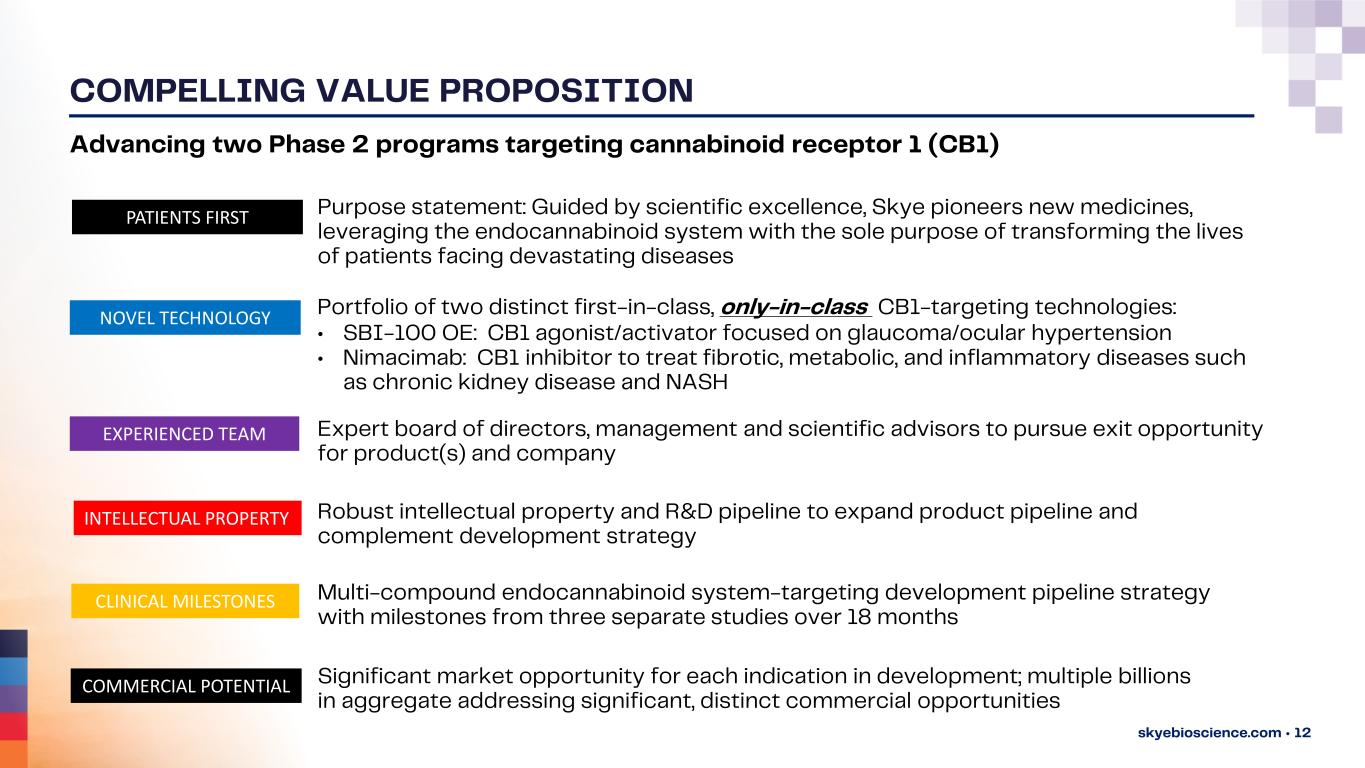
COMPELLING VALUE PROPOSITION skyebioscience.com • 12 Advancing two Phase 2 programs targeting cannabinoid receptor 1 (CB1) NOVEL TECHNOLOGY PATIENTS FIRST COMMERCIAL POTENTIAL INTELLECTUAL PROPERTY CLINICAL MILESTONES EXPERIENCED TEAM Portfolio of two distinct first-in-class, only-in-class CB1-targeting technologies: • SBI-100 OE: CB1 agonist/activator focused on glaucoma/ocular hypertension • Nimacimab: CB1 inhibitor to treat fibrotic, metabolic, and inflammatory diseases such as chronic kidney disease and NASH Expert board of directors, management and scientific advisors to pursue exit opportunity for product(s) and company Purpose statement: Guided by scientific excellence, Skye pioneers new medicines, leveraging the endocannabinoid system with the sole purpose of transforming the lives of patients facing devastating diseases Robust intellectual property and R&D pipeline to expand product pipeline and complement development strategy Multi-compound endocannabinoid system-targeting development pipeline strategy with milestones from three separate studies over 18 months Significant market opportunity for each indication in development; multiple billions in aggregate addressing significant, distinct commercial opportunities

SKYE NEXT STEPS • SBI-100 OE Phase 1 glaucoma clinical data Q3 2023 • SBI-100 OE Phase 2a glaucoma clinical trial first dosing Q4 2023 • Nimacimab Phase 2a chronic kidney disease clinical trial initiation Q1 2024 • Continued in vivo studies, biomarker development, next-generation • Planned SBI-100 OE Phase 2b glaucoma clinical trial initiation 2024 • Following P2a proof of concept for nimacimab, potential for clinical expansion to additional inflammatory, metabolic and fibrotic disorders • Maintain focused operational and clinical development strategy • Selectively evaluate business development opportunities to advance product pipeline • Plan R&D Day in fall of 2023 skyebioscience.com • 13 Achieve SBI-100 OE/glaucoma proof-of-concept milestone; advance nimacimab into clinic with longer-term view toward franchise expansion