EX-99.2
Published on February 2, 2026

Developing Innovative Medicines to Treat Obesity and Other Metabolic Diseases February 2026 Nasdaq: SKYE

© 2 02 6 Sk ye B io sc ie nc e, In c. This presentation (“Presentation”) has been prepared solely for general information purposes by or on behalf of Skye Bioscience, Inc. (together with its subsidiaries and affiliates, “Skye”). Any discussion of the potential use or expected success of our product candidates is subject to our product candidates being approved by regulatory authorities. Cautionary Language Regarding Forward-Looking Statements This Presentation includes “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. The Company intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. All statements contained in this Presentation other than statements of historical fact should be considered forward-looking statements, including, without limitation, statements relating to: Skye’s future plans and prospects; inferences or conclusions from our preclinical data; statements relating to any expectations regarding the efficacy and therapeutic potential of nimacimab as a monotherapy or in combination with semaglutide or other incretin drugs, including efficacy at higher doses based on preclinical data; the potential for future weight loss beyond 52 weeks; the timing of receipt and disclosure of data from Skye’s clinical trials; the planned timing of Skye’s anticipated milestones for nimacimab; the Company’s cash runway; future clinical development of nimacimab, including the Phase 2a extension and a potential Phase 2b clinical trial; and the regulatory strategy for nimacimab. When used herein, words including “anticipate,” “believe,” “can,” “continue,” “could,” “designed,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “might,” “plan,” “planning,” “possible,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements use these words or expressions. All forward- looking statements are based upon the Company’s current expectations and various assumptions. The Company believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. The Company may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various important risks and uncertainties, including, without limitation, the Company may not proceed into a next Phase 2 clinical trial for nimacimab, including due to capital constraints and program considerations; the indicated trajectory of continuing weight loss at 52 weeks may not ultimately be observed; it is possible that higher dosing will produce adversely different safety and tolerability results than those observed to date; the Company’s dependence on third parties in connection with product manufacturing; research and preclinical and clinical testing; the Company’s ability to advance, obtain regulatory approval of and ultimately commercialize nimacimab, competitive products or approaches limiting the commercial value of nimacimab; the timing and results of preclinical and clinical trials; the Company’s ability to fund development activities and achieve development goals; the impact of any global pandemics, inflation, supply chain issues, government shutdowns, high interest rates, adverse regulatory changes; the Company’s ability to protect its intellectual property; risks associated with the Company’s common stock and the other important factors discussed under the caption “Risk Factors” in the Company’s filings with the Securities and Exchange Commission, including in its Annual Report on Form 10-K for the year ended December 31, 2024, which are accessible on the SEC’s website at www.sec.gov and the Investors section of the Company’s website. Any such forward- looking statements represent management’s estimates as of the date of this Presentation. While the Company may elect to update such forward-looking statements at some point in the future, except as required by law, it disclaims any obligation to do so, even if subsequent events cause the Company’s views to change. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this Presentation. Industry and Market Data, and Third-Party Reports The views and statements provided in this Presentation are based on information derived from Skye’s internal estimates and research, studies, publications, surveys and other information provided by third parties and also from publicly available sources. In this Presentation, Skye relies on, and refers to, publicly available information and statistics regarding market participants in the sector in which Skye competes and other industry data. Any comparison of Skye to any other entity assumes the reliability of the information available to Skye. Skye has not independently verified the accuracy or completeness of these sources. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources. Skye has not independently verified, and makes no representation as to, the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. All of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Disclaimer and Important Information for Investors 2 Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l.
© 2 02 6 Sk ye B io sc ie nc e, In c. Executive Summary Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 3 Nimacimab Semaglutid e Additive Weight Loss Favorable Tolerability Profile CBeyond data established potential path for combination strategy with existing incretin therapies Skye has developed a combination strategy for nimacimab + semaglutide that has the potential to provide a compelling new therapeutic option for patients. 52-Week Combination Data High-Dose Rationale High-Dose Feasibility Regulatory Alignment Commercial Scalability Attractive Target Product Profile Improved Durability of Weight Loss
© 2 02 6 Sk ye B io sc ie nc e, In c. Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 4 Nimacimab: complementary, not competitive – potential scalable add-on to enhance incretin therapy • Additive efficacy on top of GLP-1s: 13% weight-loss at 26 weeks, which was significantly better than semaglutide alone (p=0.03) in the Phase 2a study; 22.3% total weight loss at 52 weeks based on interim Phase 2a extension study data, with no plateau observed at 52 weeks. • Durable & quality weight loss: Lower post-treatment rebound (~17.8% combo vs. ~37.3% semaglutide-alone) and a favorable body-composition profile (increases fat loss by 37% compared to semaglutide alone) in Phase 2a study supporting potential use for maintenance after incretins and minimizing impact of weight regain. • Safe combo, titration-free: No additive GI burden and 0% neuropsychiatric AEs in the combo arm in Phase 2a study; enables potential straightforward combination use across the incretin class. • Potential differentiated mechanism in a crowded indication; potential first in class combination profile: In a crowded incretin landscape, peripheral CB1 mechanism provides potential differentiation that we believe is complementary to incretins. Investment Thesis
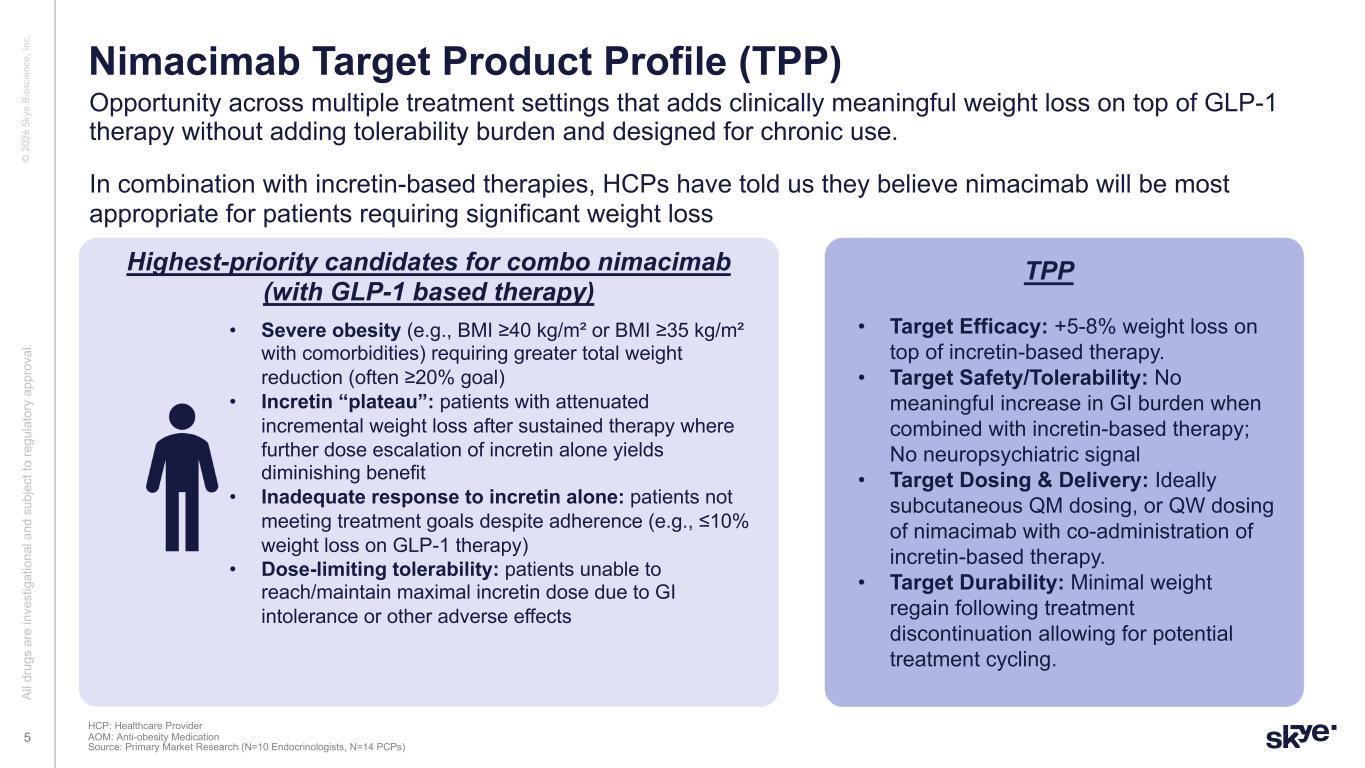
© 2 02 6 Sk ye B io sc ie nc e, In c. Opportunity across multiple treatment settings that adds clinically meaningful weight loss on top of GLP-1 therapy without adding tolerability burden and designed for chronic use. Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 5 Nimacimab Target Product Profile (TPP) HCP: Healthcare Provider AOM: Anti-obesity Medication Source: Primary Market Research (N=10 Endocrinologists, N=14 PCPs) In combination with incretin-based therapies, HCPs have told us they believe nimacimab will be most appropriate for patients requiring significant weight loss Highest-priority candidates for combo nimacimab (with GLP-1 based therapy) • Severe obesity (e.g., BMI ≥40 kg/m² or BMI ≥35 kg/m² with comorbidities) requiring greater total weight reduction (often ≥20% goal) • Incretin “plateau”: patients with attenuated incremental weight loss after sustained therapy where further dose escalation of incretin alone yields diminishing benefit • Inadequate response to incretin alone: patients not meeting treatment goals despite adherence (e.g., ≤10% weight loss on GLP-1 therapy) • Dose-limiting tolerability: patients unable to reach/maintain maximal incretin dose due to GI intolerance or other adverse effects • Target Efficacy: +5-8% weight loss on top of incretin-based therapy. • Target Safety/Tolerability: No meaningful increase in GI burden when combined with incretin-based therapy; No neuropsychiatric signal • Target Dosing & Delivery: Ideally subcutaneous QM dosing, or QW dosing of nimacimab with co-administration of incretin-based therapy. • Target Durability: Minimal weight regain following treatment discontinuation allowing for potential treatment cycling. TPP

© 2 02 6 Sk ye B io sc ie nc e, In c. Presentation Overview 1.0 Nimacimab Overview – A Highly Peripherally-restricted CB1-inhibiting Antibody 2.0 CBeyond Extension Data Review – 52-week Combination Data 2.1 CBeyond – What We Learned 3.0 CBeyond2 – Phase 2b Adaptive Design Dose Ranging Study 3.1 Regulatory Strategy – Combination Approval, Monotherapy Opportunity 4.0 Financial Overview and Team Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 6

Nimacimab A Highly Peripherally-restricted CB1-inhibiting Antibody that Stands Apart from Small– molecule CB1 Inhibitors 1.0
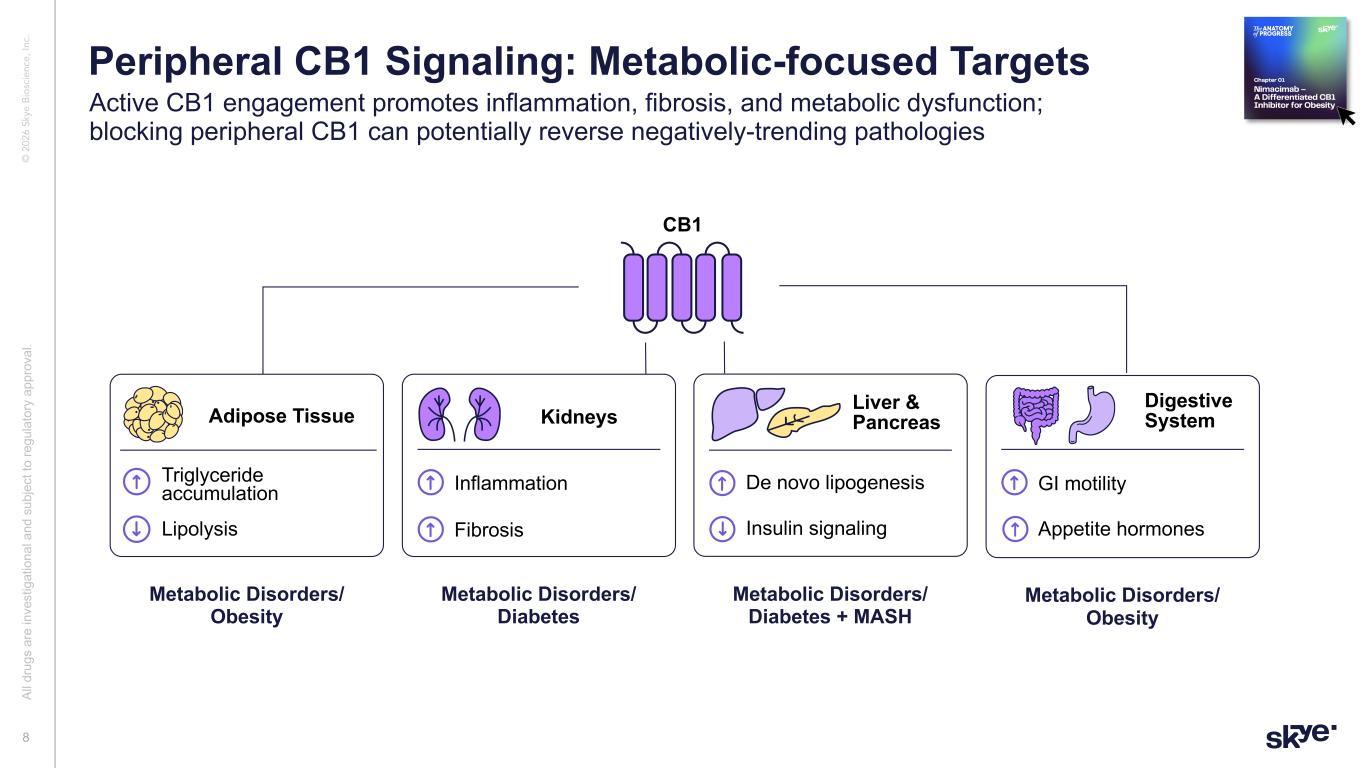
© 2 02 6 Sk ye B io sc ie nc e, In c. Metabolic Disorders/ Obesity Adipose Tissue CB1 Triglyceride accumulation Lipolysis Kidneys Inflammation Fibrosis Metabolic Disorders/ Diabetes Liver & Pancreas De novo lipogenesis Insulin signaling Metabolic Disorders/ Diabetes + MASH Digestive System GI motility Appetite hormones Metabolic Disorders/ Obesity Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 8 Peripheral CB1 Signaling: Metabolic-focused Targets Active CB1 engagement promotes inflammation, fibrosis, and metabolic dysfunction; blocking peripheral CB1 can potentially reverse negatively-trending pathologies
© 2 02 6 Sk ye B io sc ie nc e, In c. Long Half-life Potential Safe & Effective Drug Exclusion from Brain • Stable antibody with half-life of 18-21 days (potential Q2W or monthly dosing) • Single mutation in the hinge region that prevents antibody Fab exchange • Multiple NHP studies: background levels in CNS/brain (even at high doses) • No accumulation of antibody in CNS/brain despite multiple weekly doses • NOAEL > 75 mg/kg. MTD not reached • Achieve ~7x peripheral CB1 inhibition while ~600x below CB1 inhibition in brain • Allosteric binding maintains peripheral CB1 inhibition with increased endocannabinoids • Supports a favorable therapeutic index to safely and effectively treat obesity Differentiated Inhibitor • Functions as both an antagonist and an inverse agonist • Binds allosteric site and non-competitively inhibits CB1, independent of agonist 9 Nimacimab: Peripherally-restricted CB1-inhibiting Antibody Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l.
© 2 02 6 Sk ye B io sc ie nc e, In c. 2-AG or AEA Negative Allosteric Modulator Significantly less brain penetration than small molecules currently in development Unlike small molecules currently in development, nimacimab retains potency even in the presence of competition Peripheral Restriction Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 10 Nimacimab’s Potential Differentiation from Small Molecule CB1 Inhibitors
© 2 02 6 Sk ye B io sc ie nc e, In c. Peripheral Modulation of Appetite Regulating Hormones GLP-1 Improvement and Restoration of Glycemic Control Reduced fasting insulin and improved glucose control Enhanced Lipid Metabolism Decreased steatosis and serum cholesterol Reduction of Obesity- Induced Inflammation Decreased inflammation and fibrotic markers 01 02 03 04 Leptin Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 11 Four Mechanistic Pillars of Nimacimab

2.0 Review of Interim Extension Study: 52-Week Combination Data
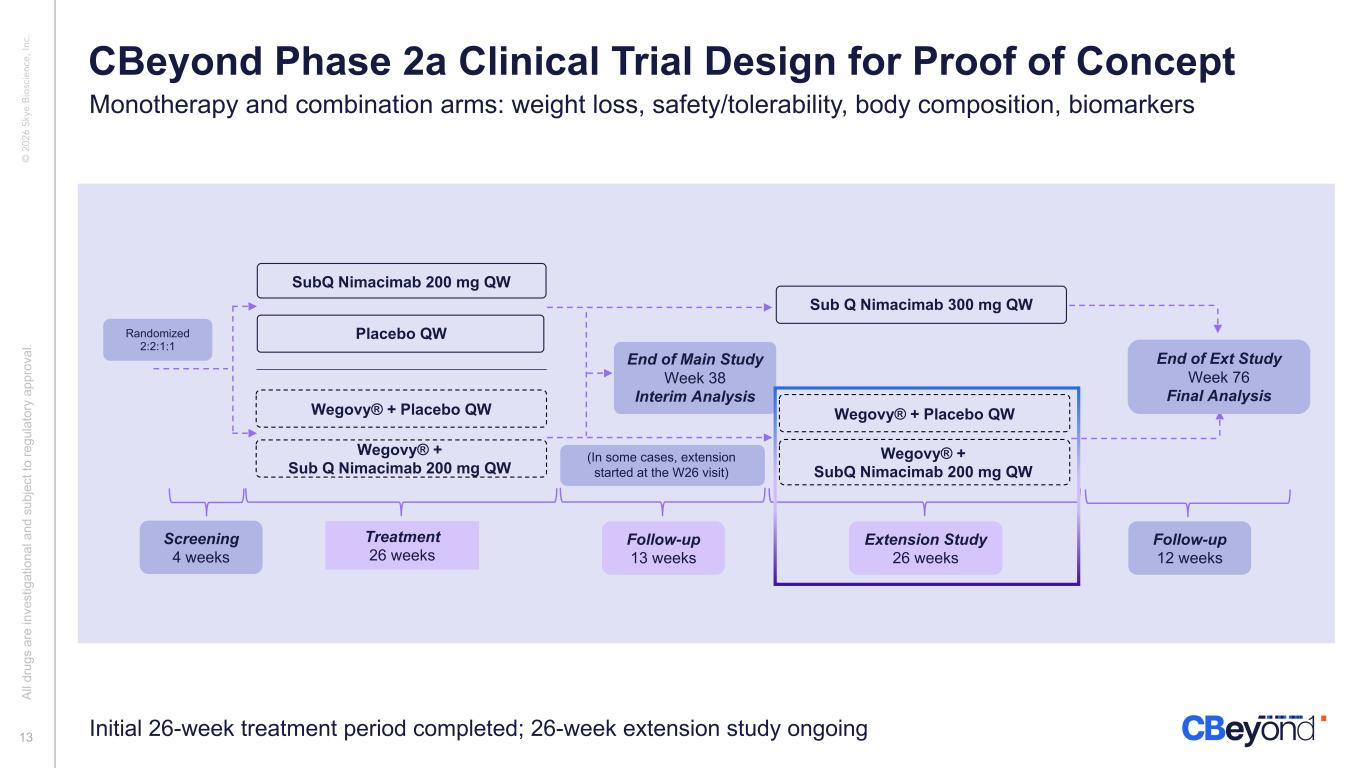
© 2 02 6 Sk ye B io sc ie nc e, In c. SubQ Nimacimab 200 mg QW Treatment 26 weeks Follow-up 13 weeks End of Main Study Week 38 Interim Analysis Placebo QW Wegovy® + Placebo QW Wegovy® + Sub Q Nimacimab 200 mg QW Extension Study 26 weeks Follow-up 12 weeks Wegovy® + SubQ Nimacimab 200 mg QW Sub Q Nimacimab 300 mg QW End of Ext Study Week 76 Final Analysis Wegovy® + Placebo QW Screening 4 weeks (In some cases, extension started at the W26 visit) Initial 26-week treatment period completed; 26-week extension study ongoing Randomized 2:2:1:1 Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 13 CBeyond Phase 2a Clinical Trial Design for Proof of Concept Monotherapy and combination arms: weight loss, safety/tolerability, body composition, biomarkers
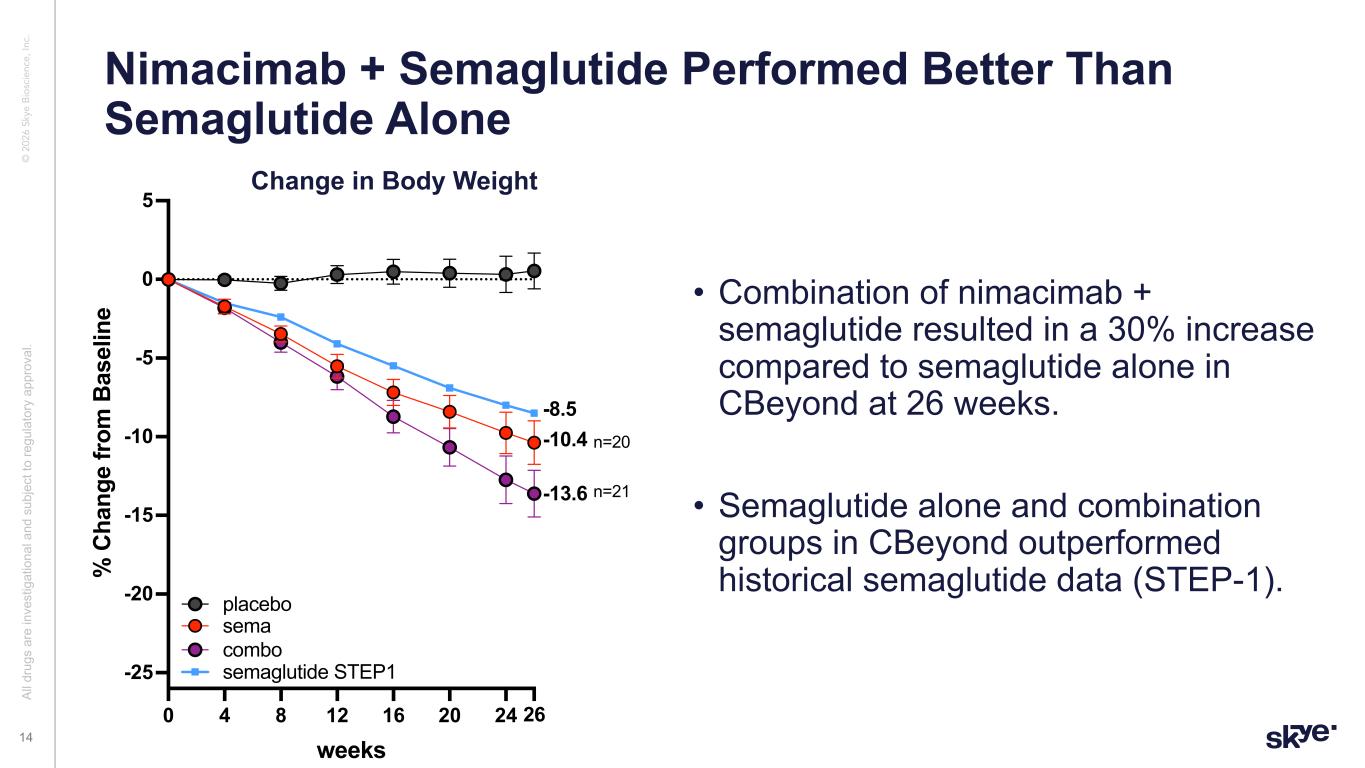
© 2 02 6 Sk ye B io sc ie nc e, In c. • Combination of nimacimab + semaglutide resulted in a 30% increase compared to semaglutide alone in CBeyond at 26 weeks. • Semaglutide alone and combination groups in CBeyond outperformed historical semaglutide data (STEP-1). Nimacimab + Semaglutide Performed Better Than Semaglutide Alone Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 14 0 4 8 12 16 20 24 26 -25 -20 -15 -10 -5 0 5 weeks % C ha ng e fr om B as el in e combo + sema up to wk26 + STEP1 placebo sema combo -10.4 -13.6 n=20 n=21 semaglutide STEP1 -8.5 Change in Body Weight
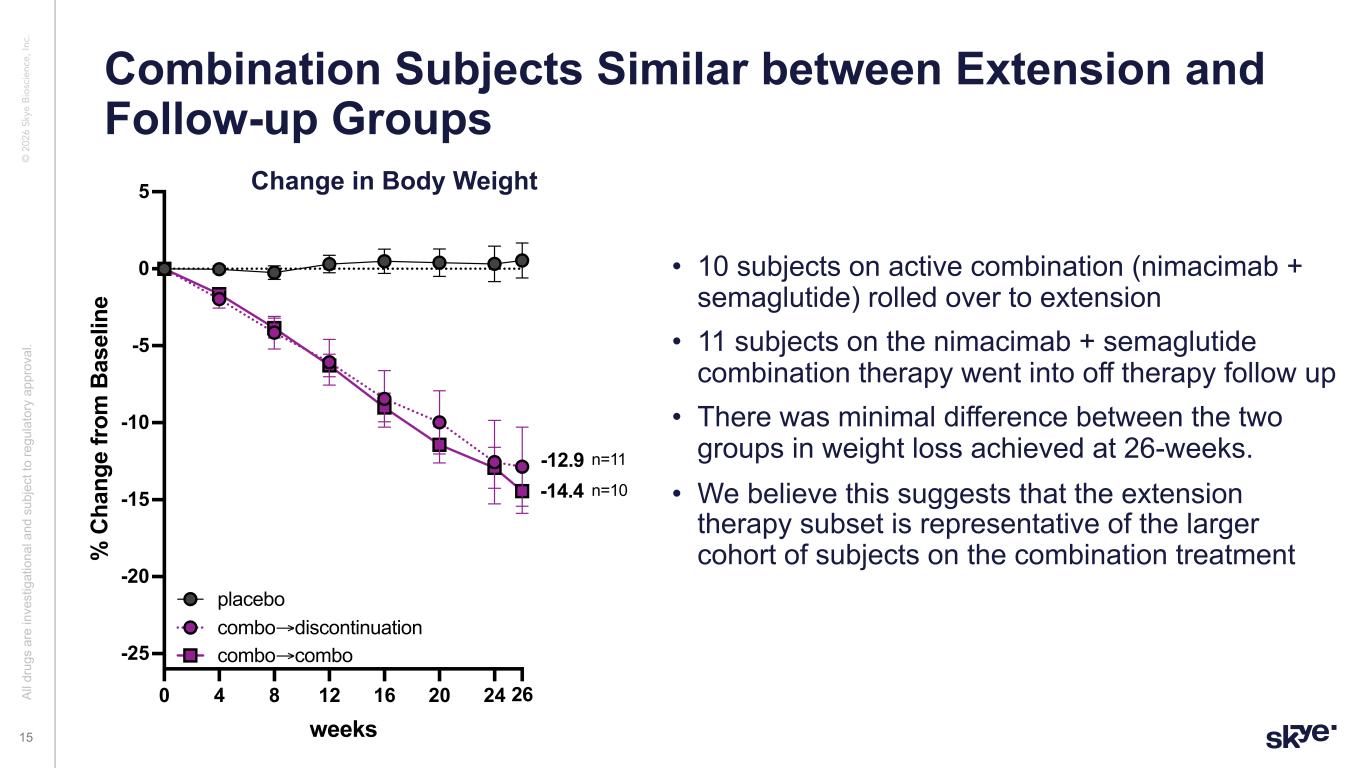
© 2 02 6 Sk ye B io sc ie nc e, In c. • 10 subjects on active combination (nimacimab + semaglutide) rolled over to extension • 11 subjects on the nimacimab + semaglutide combination therapy went into off therapy follow up • There was minimal difference between the two groups in weight loss achieved at 26-weeks. • We believe this suggests that the extension therapy subset is representative of the larger cohort of subjects on the combination treatment Combination Subjects Similar between Extension and Follow-up Groups Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 15 0 4 8 12 16 20 24 26 -25 -20 -15 -10 -5 0 5 weeks % C ha ng e fr om B as el in e combo 2 groups combo→discontinuation placebo combo→combo -14.4 -12.9 n=11 n=10 Change in Body Weight
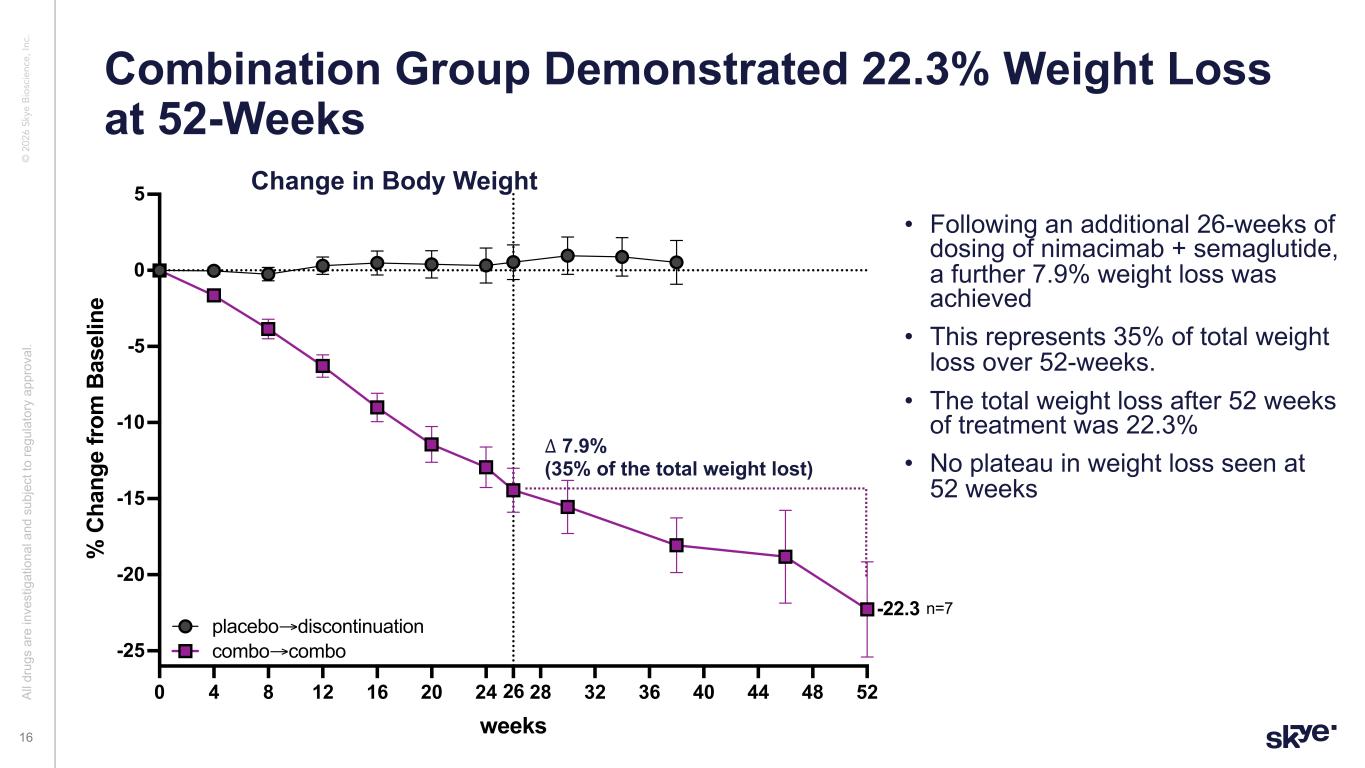
© 2 02 6 Sk ye B io sc ie nc e, In c. • Following an additional 26-weeks of dosing of nimacimab + semaglutide, a further 7.9% weight loss was achieved • This represents 35% of total weight loss over 52-weeks. • The total weight loss after 52 weeks of treatment was 22.3% • No plateau in weight loss seen at 52 weeks Combination Group Demonstrated 22.3% Weight Loss at 52-Weeks Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 16 0 4 8 12 16 20 24 28 32 36 40 44 48 5226 -25 -20 -15 -10 -5 0 5 weeks % C ha ng e fr om B as el in e combo extension placebo→discontinuation combo→combo -22.3 n=7 ∆ 7.9% (35% of the total weight lost) Change in Body Weight
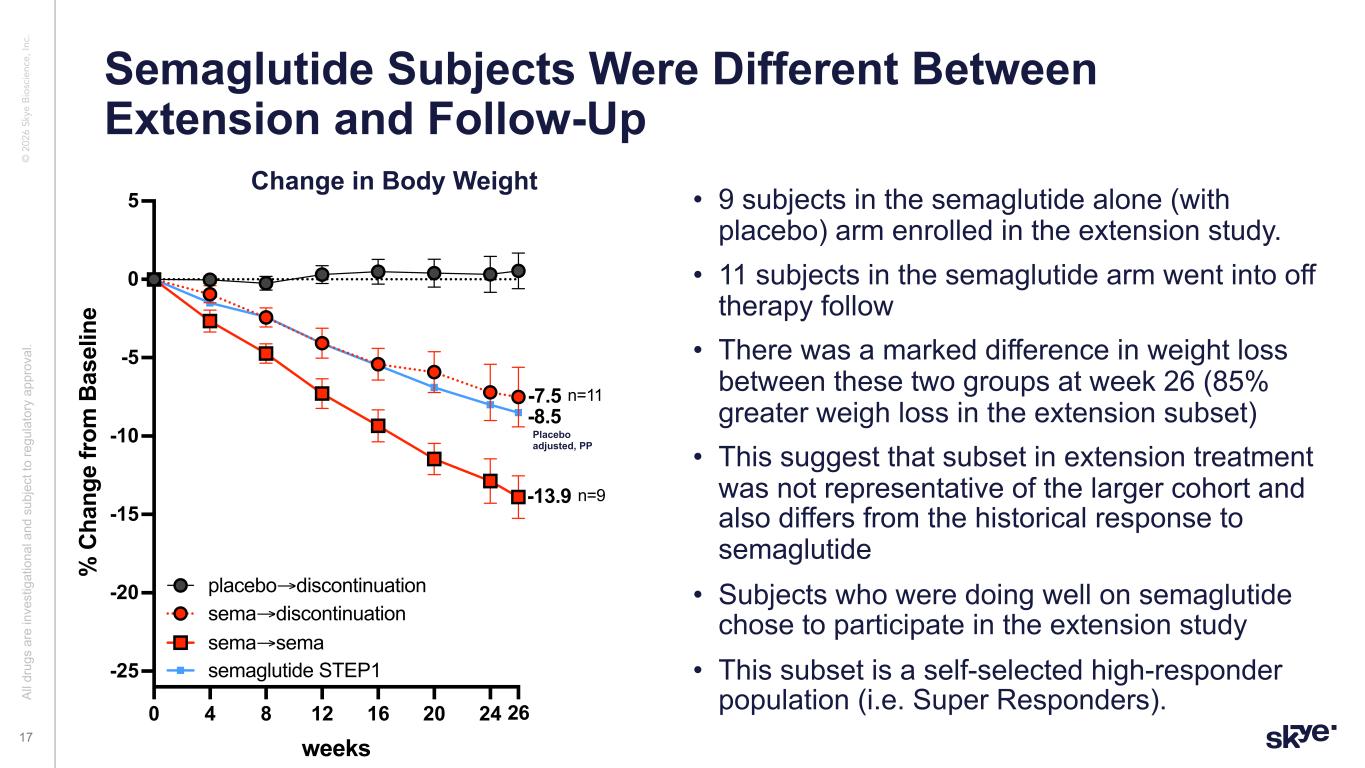
© 2 02 6 Sk ye B io sc ie nc e, In c. • 9 subjects in the semaglutide alone (with placebo) arm enrolled in the extension study. • 11 subjects in the semaglutide arm went into off therapy follow • There was a marked difference in weight loss between these two groups at week 26 (85% greater weigh loss in the extension subset) • This suggest that subset in extension treatment was not representative of the larger cohort and also differs from the historical response to semaglutide • Subjects who were doing well on semaglutide chose to participate in the extension study • This subset is a self-selected high-responder population (i.e. Super Responders). Semaglutide Subjects Were Different Between Extension and Follow-Up Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 17 Change in Body Weight 0 4 8 12 16 20 24 26 -25 -20 -15 -10 -5 0 5 weeks % C ha ng e fr om B as el in e sema extension + rebound wk26 +STEP sema→discontinuation placebo→discontinuation sema→sema -7.5 -13.9 n=11 n=9 semaglutide STEP1 -8.5 Placebo adjusted, PP
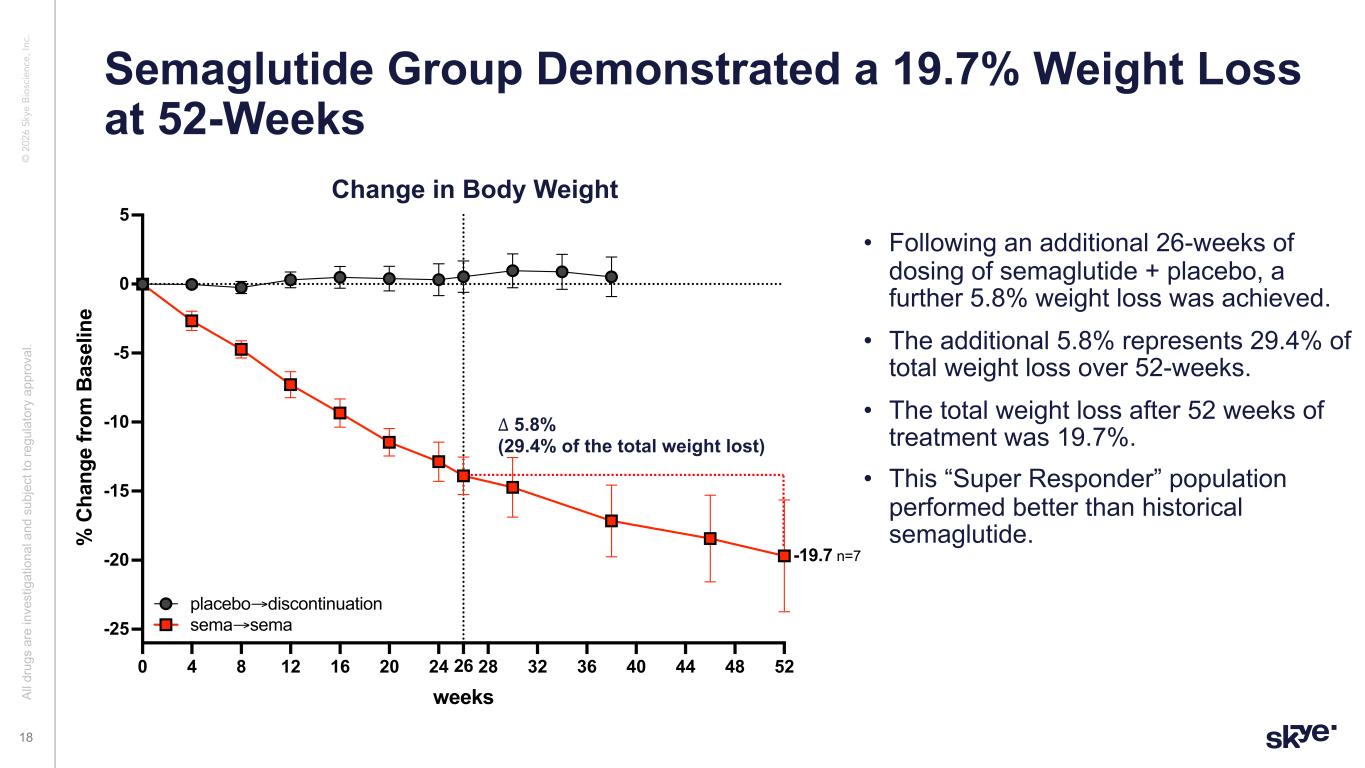
© 2 02 6 Sk ye B io sc ie nc e, In c. • Following an additional 26-weeks of dosing of semaglutide + placebo, a further 5.8% weight loss was achieved. • The additional 5.8% represents 29.4% of total weight loss over 52-weeks. • The total weight loss after 52 weeks of treatment was 19.7%. • This “Super Responder” population performed better than historical semaglutide. Semaglutide Group Demonstrated a 19.7% Weight Loss at 52-Weeks Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 18 0 4 8 12 16 20 24 28 32 36 40 44 48 5226 -25 -20 -15 -10 -5 0 5 weeks % C ha ng e fr om B as el in e sema extension placebo→discontinuation sema→sema -19.7 n=7 ∆ 5.8% (29.4% of the total weight lost) Change in Body Weight
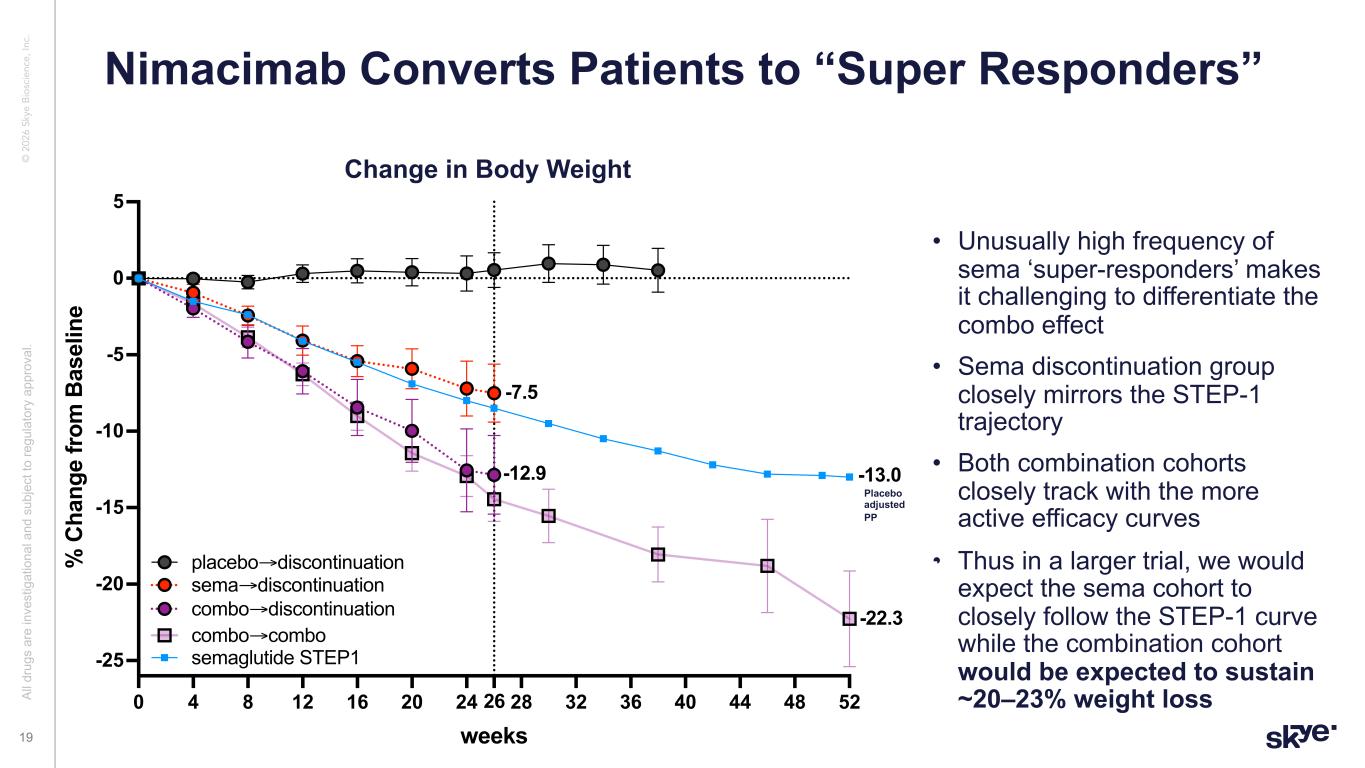
© 2 02 6 Sk ye B io sc ie nc e, In c. • Unusually high frequency of sema ‘super-responders’ makes it challenging to differentiate the combo effect • Sema discontinuation group closely mirrors the STEP-1 trajectory • Both combination cohorts closely track with the more active efficacy curves • Thus in a larger trial, we would expect the sema cohort to closely follow the STEP-1 curve while the combination cohort would be expected to sustain ~20–23% weight loss Nimacimab Converts Patients to “Super Responders” Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 19 Change in Body Weight Placebo adjusted PP n=7 0 4 8 12 16 20 24 28 32 36 40 44 48 5226 -25 -20 -15 -10 -5 0 5 weeks % C ha ng e fr om B as el in e Copy of rebound up to wk26 and extrebound extension and STEP1 veh adjusted sema→discontinuation combo→discontinuation placebo→discontinuation semaglutide STEP1 -22.3 -13.0 combo→combo -7.5 -12.9
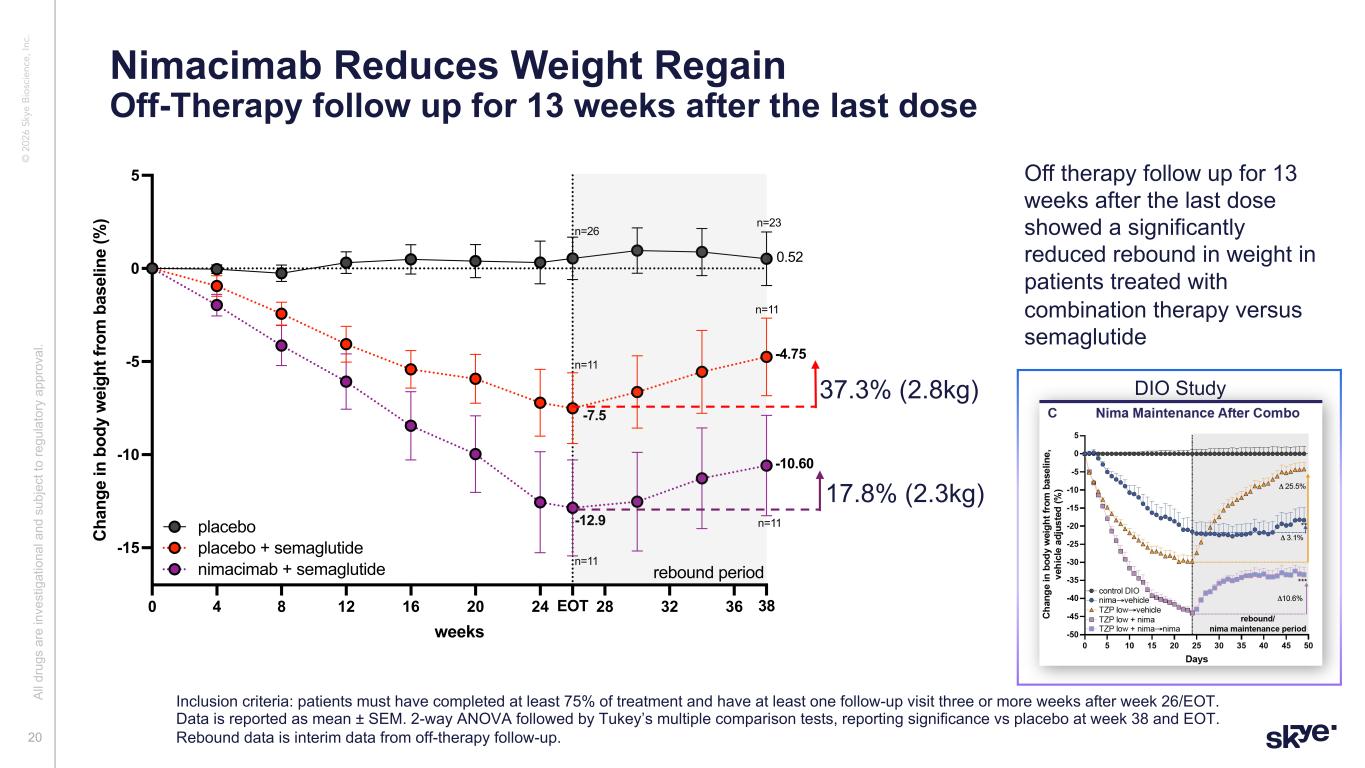
© 2 02 6 Sk ye B io sc ie nc e, In c. Nimacimab Reduces Weight Regain Off-Therapy follow up for 13 weeks after the last dose Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. Inclusion criteria: patients must have completed at least 75% of treatment and have at least one follow-up visit three or more weeks after week 26/EOT. Data is reported as mean ± SEM. 2-way ANOVA followed by Tukey’s multiple comparison tests, reporting significance vs placebo at week 38 and EOT. Rebound data is interim data from off-therapy follow-up. 20 0 4 8 12 16 20 24 28 32 36EOT 38 -15 -10 -5 0 5 weeks C ha ng e in b od y w ei gh t f ro m b as el in e (% ) rebound combos n=11: rebound combos placebo + semaglutide nimacimab + semaglutide placebo 0.52 -4.75 -10.60 n=11 n=11 n=11 n=11 n=23 n=26 -7.5 -12.9 rebound period 37.3% (2.8kg) 17.8% (2.3kg) Off therapy follow up for 13 weeks after the last dose showed a significantly reduced rebound in weight in patients treated with combination therapy versus semaglutide DIO Study
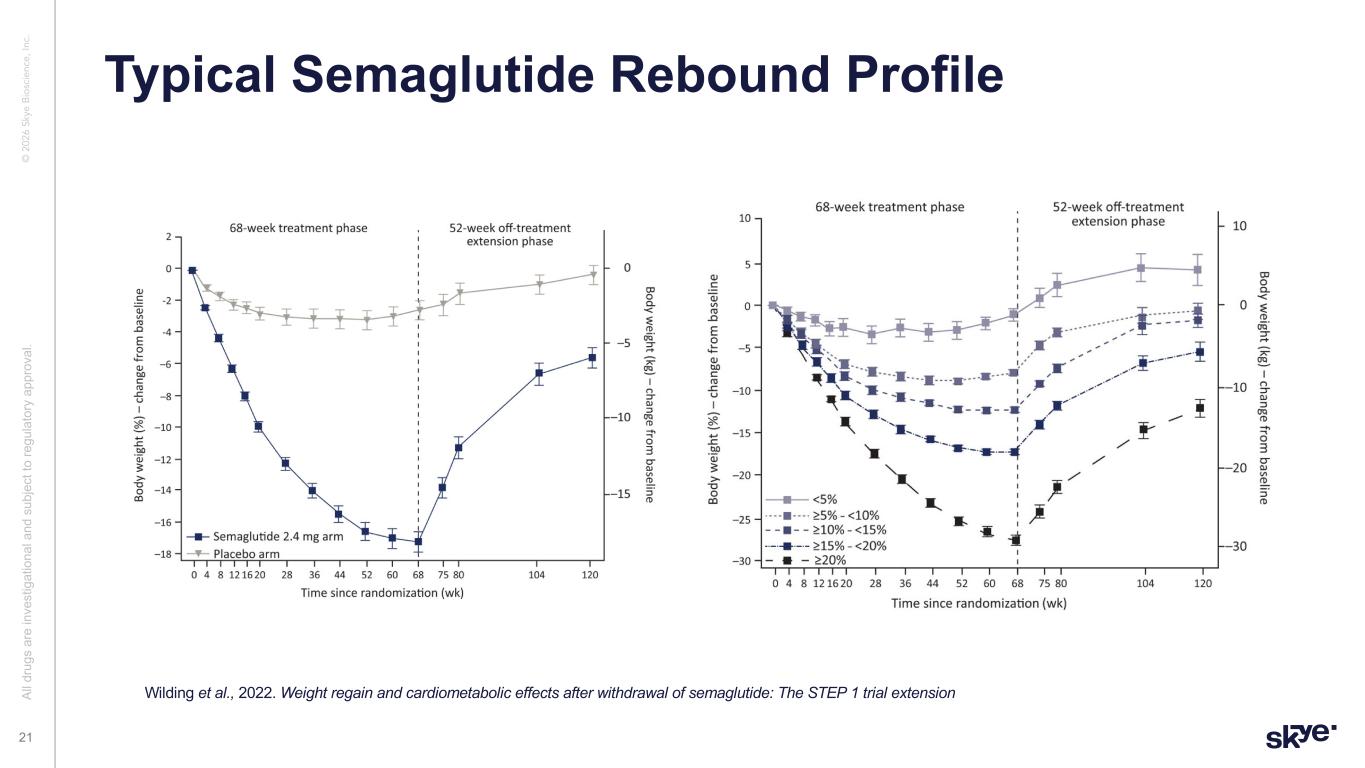
© 2 02 6 Sk ye B io sc ie nc e, In c. Typical Semaglutide Rebound Profile Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 21 Wilding et al., 2022. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension
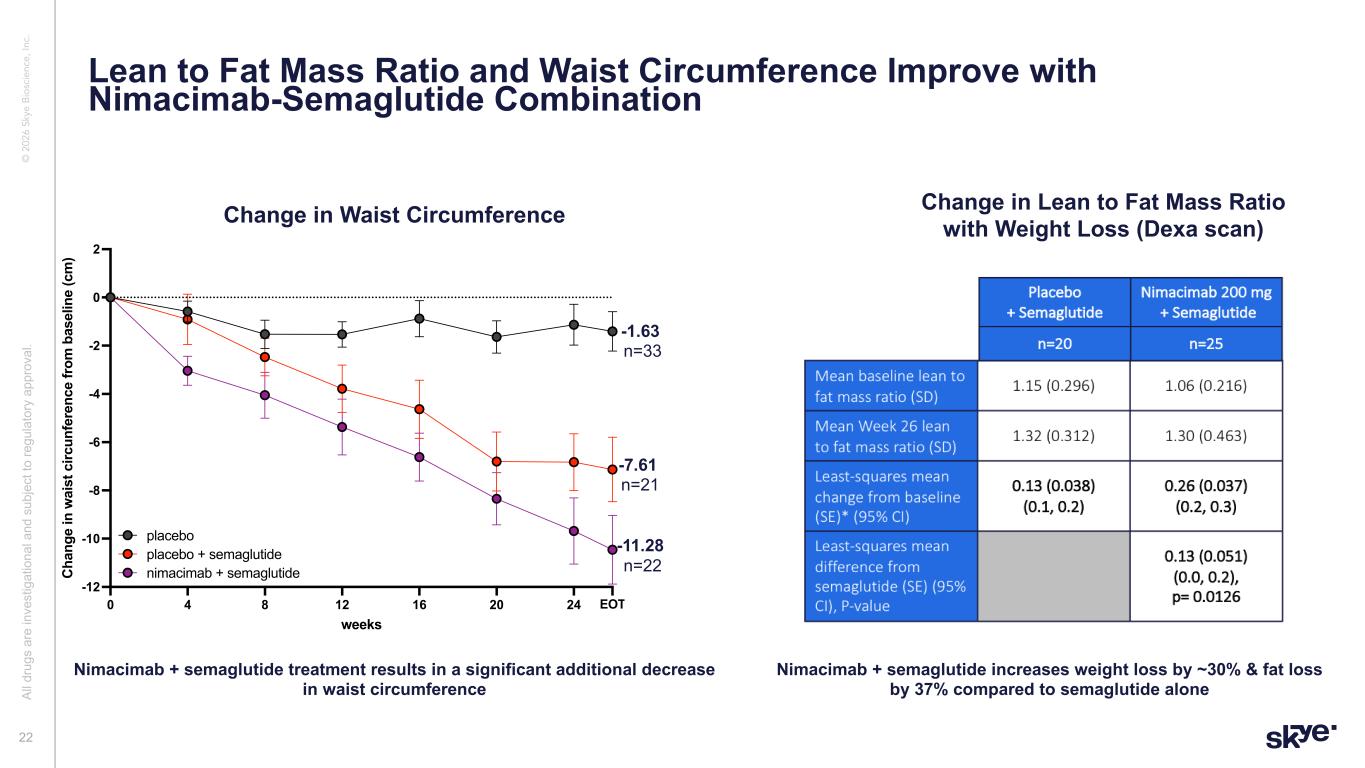
© 2 02 6 Sk ye B io sc ie nc e, In c. Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 22 Lean to Fat Mass Ratio and Waist Circumference Improve with Nimacimab-Semaglutide Combination Nimacimab + semaglutide increases weight loss by ~30% & fat loss by 37% compared to semaglutide alone Change in Lean to Fat Mass Ratio with Weight Loss (Dexa scan) 0 4 8 12 16 20 24 EOT -12 -10 -8 -6 -4 -2 0 2 weeks C ha ng e in w ai st c irc un fe re nc e fr om b as el in e (c m ) %change in WC sema combo placebo placebo placebo + semaglutide nimacimab + semaglutide -1.4 -7.1 -10.5 n=33 n=21 n=22 -1.63 n=33 -7.61 n=21 -1 .28 n=22 Nimacimab + semaglutide treatment results in a significant additional decrease in waist circumference Change in Waist Circumference
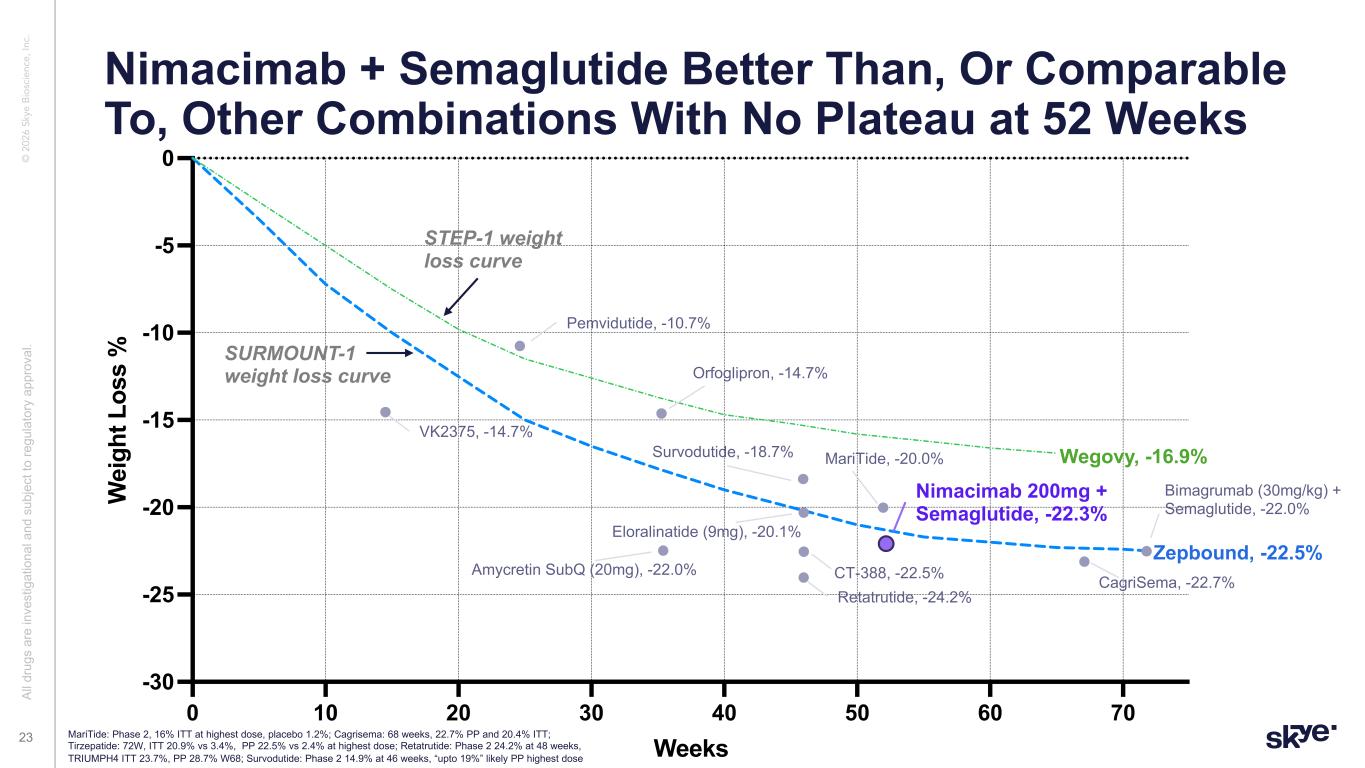
© 2 02 6 Sk ye B io sc ie nc e, In c. Nimacimab + Semaglutide Better Than, Or Comparable To, Other Combinations With No Plateau at 52 Weeks Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 23 0 10 20 30 40 50 60 70 -30 -25 -20 -15 -10 -5 0 Weeks W ei gh t L os s % Wegovy, -16.9% Zepbound, -22.5% Nimacimab 200mg + Semaglutide, -22.3% Retatrutide, -24.2% Eloralinatide (9mg), -20.1% CT-388, -22.5% CagriSema, -22.7% Orfoglipron, -14.7% MariTide, -20.0%Survodutide, -18.7% Bimagrumab (30mg/kg) + Semaglutide, -22.0% STEP-1 weight loss curve SURMOUNT-1 weight loss curve Amycretin SubQ (20mg), -22.0% VK2375, -14.7% Pemvidutide, -10.7% MariTide: Phase 2, 16% ITT at highest dose, placebo 1.2%; Cagrisema: 68 weeks, 22.7% PP and 20.4% ITT; Tirzepatide: 72W, ITT 20.9% vs 3.4%, PP 22.5% vs 2.4% at highest dose; Retatrutide: Phase 2 24.2% at 48 weeks, TRIUMPH4 ITT 23.7%, PP 28.7% W68; Survodutide: Phase 2 14.9% at 46 weeks, “upto 19%” likely PP highest dose
© 2 02 6 Sk ye B io sc ie nc e, In c. • We believe semaglutide-alone group self-selected for a “Super Responder” population which elected to continue enrollment in the 26-week extension study and showed continued weight loss beyond what is typically seen with semaglutide therapy alone. • Nimacimab has demonstrated the ability to increase weight loss when combined with semaglutide by converting patients to “Super Responders”. • Combination of nimacimab + semaglutide continued to demonstrate safe and tolerable profile at tested doses, similar to semaglutide- alone. • Weight loss of 22.3% with no evidence of plateau is comparable to other combination therapies at 52 weeks. Summary and Conclusions Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 24

2.1 What We Learned From Dosing Strategy and Rationale

© 2 02 6 Sk ye B io sc ie nc e, In c. Data from CBeyond, together with our preclinical models, suggests: 1. Concentration in the serum does not equal concentration in the tissues, and achieving a higher serum concentration and steady-state sooner through a loading dose can potentially improve response. 2. Nimacimab can be dosed significantly higher and drive additional weight loss as both a monotherapy and in combination with semaglutide. 3. Compliance and retention are priorities for the success of the next study. We intend to include weekly on-site visits for dosing and implement highly competitive retention programs. What We Learned from CBeyond Regarding Dosing Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 26
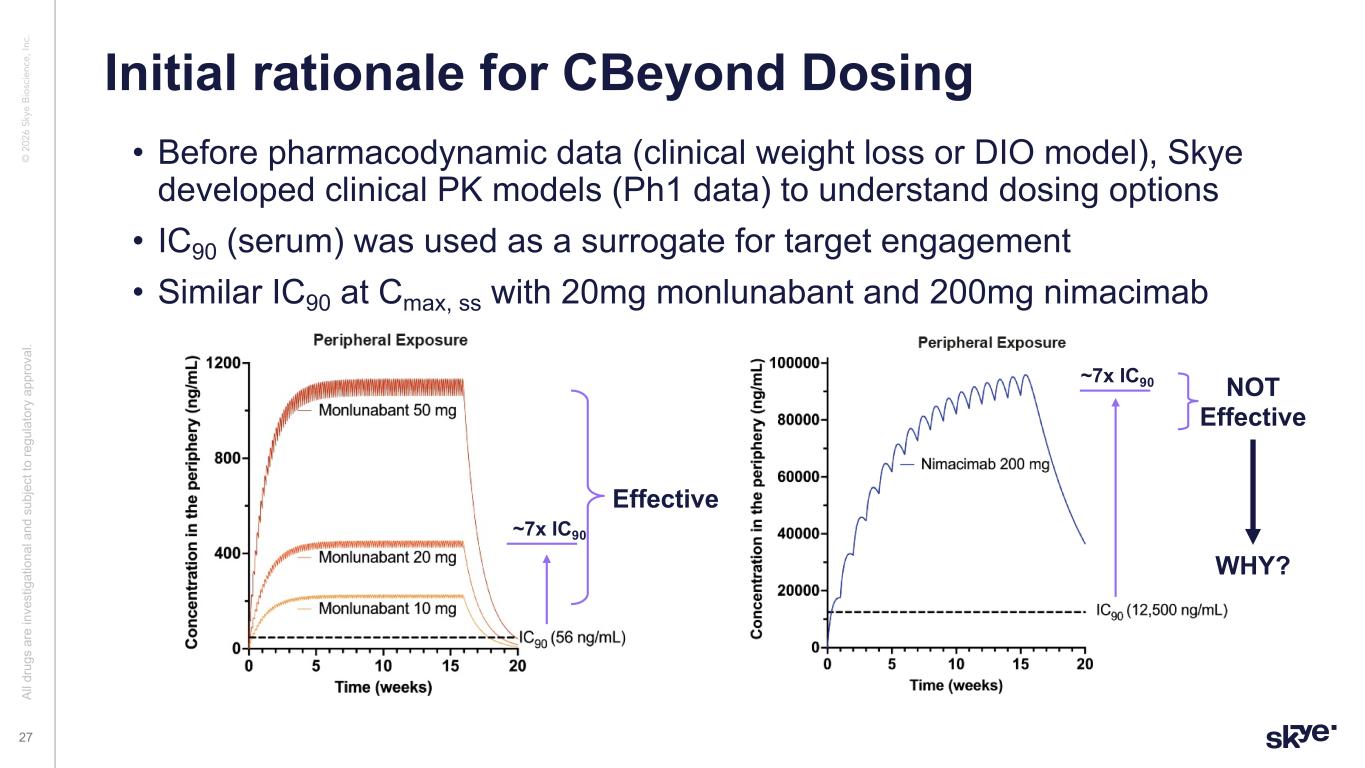
© 2 02 6 Sk ye B io sc ie nc e, In c. Initial rationale for CBeyond Dosing Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 27 • Before pharmacodynamic data (clinical weight loss or DIO model), Skye developed clinical PK models (Ph1 data) to understand dosing options • IC90 (serum) was used as a surrogate for target engagement • Similar IC90 at Cmax, ss with 20mg monlunabant and 200mg nimacimab ~7x IC90 ~7x IC90 Effective NOT Effective WHY?
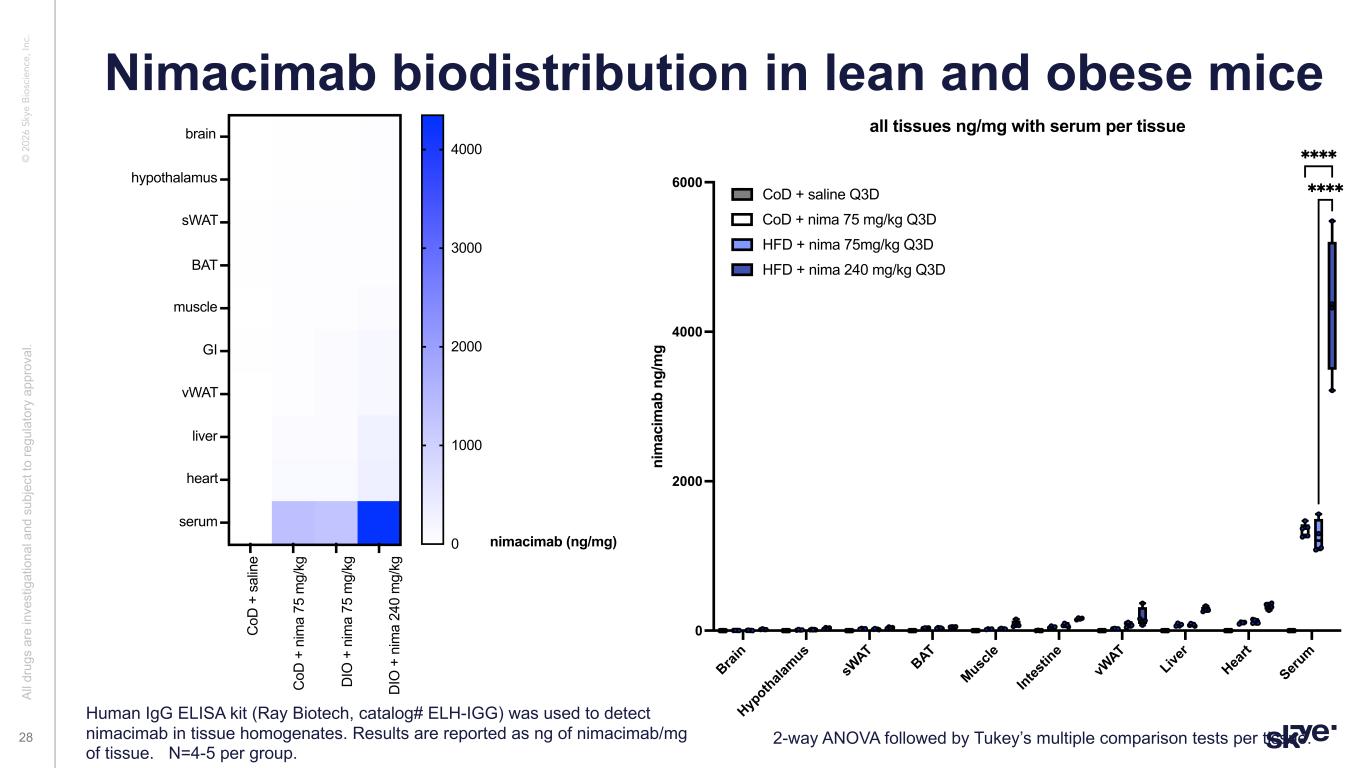
© 2 02 6 Sk ye B io sc ie nc e, In c. Nimacimab biodistribution in lean and obese mice Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 28 2-way ANOVA followed by Tukey’s multiple comparison tests per tissue. Human IgG ELISA kit (Ray Biotech, catalog# ELH-IGG) was used to detect nimacimab in tissue homogenates. Results are reported as ng of nimacimab/mg of tissue. N=4-5 per group. Brai n Hyp othala mus sW AT BAT Musc le Intes tin e vW AT Live r Hea rt Seru m 0 2000 4000 6000 ni m ac im ab n g/ m g all tissues ng/mg with serum per tissue CoD + saline Q3D CoD + nima 75 mg/kg Q3D HFD + nima 75mg/kg Q3D HFD + nima 240 mg/kg Q3D ✱✱✱✱ ✱✱✱✱ A B C D 1 2 3 4 5 6 7 8 9 10 0 1000 2000 3000 muscle heart GI liver brain nimacimab (ng/mg) hypothalamus BAT sWAT vWAT C oD + s al in e C oD + n im a 75 m g/ kg D IO + n im a 75 m g/ kg D IO + n im a 24 0 m g/ kg 4000 serum
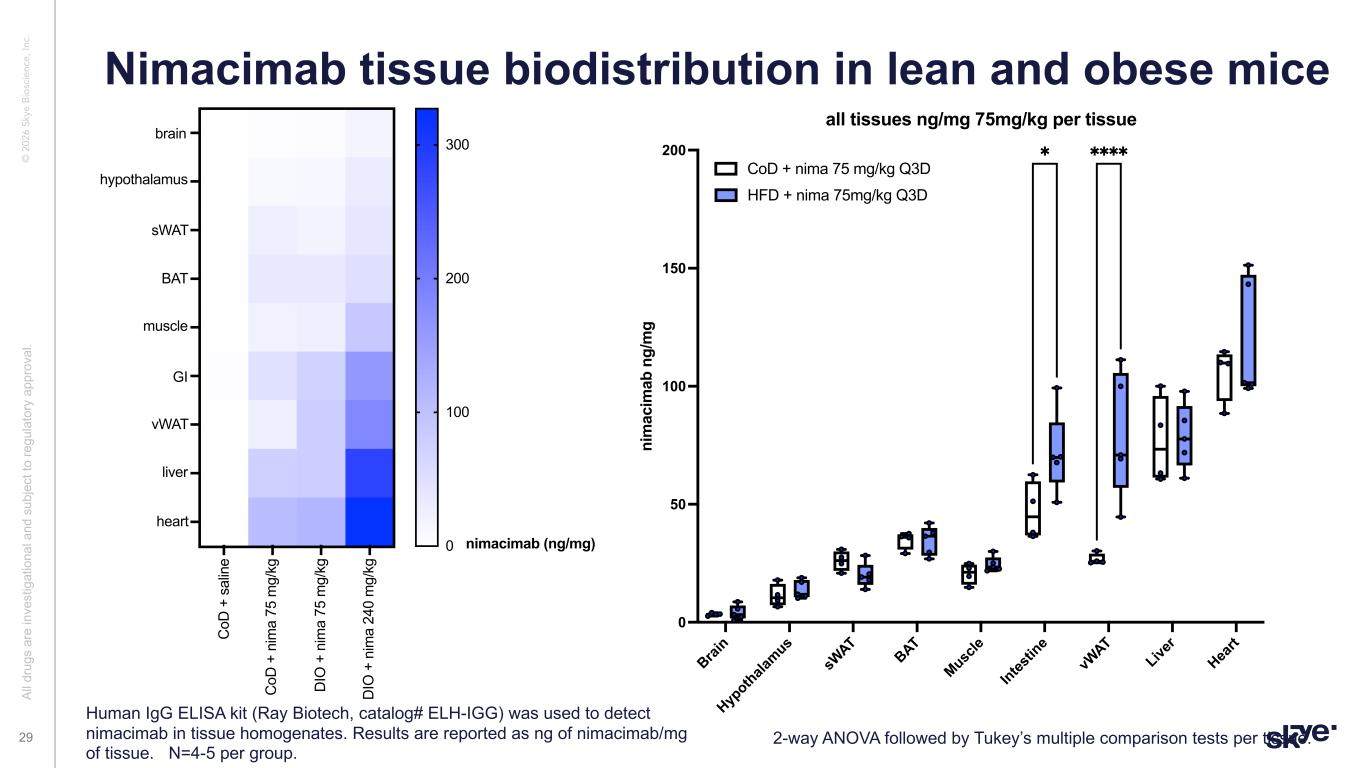
© 2 02 6 Sk ye B io sc ie nc e, In c. Nimacimab tissue biodistribution in lean and obese mice Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 29 A B C D 1 2 3 4 5 6 7 8 9 0 100 200 300 muscle heart GI liver brain nimacimab (ng/mg) hypothalamus BAT sWAT vWAT C oD + s al in e C oD + n im a 75 m g/ kg D IO + n im a 75 m g/ kg D IO + n im a 24 0 m g/ kg Brai n Hyp othala mus sW AT BAT Musc le Intes tin e vW AT Live r Hea rt 0 50 100 150 200 ni m ac im ab n g/ m g all tissues ng/mg 75mg/kg per tissue CoD + nima 75 mg/kg Q3D HFD + nima 75mg/kg Q3D ✱ ✱✱✱✱ 2-way ANOVA followed by Tukey’s multiple comparison tests per tissue. Human IgG ELISA kit (Ray Biotech, catalog# ELH-IGG) was used to detect nimacimab in tissue homogenates. Results are reported as ng of nimacimab/mg of tissue. N=4-5 per group.
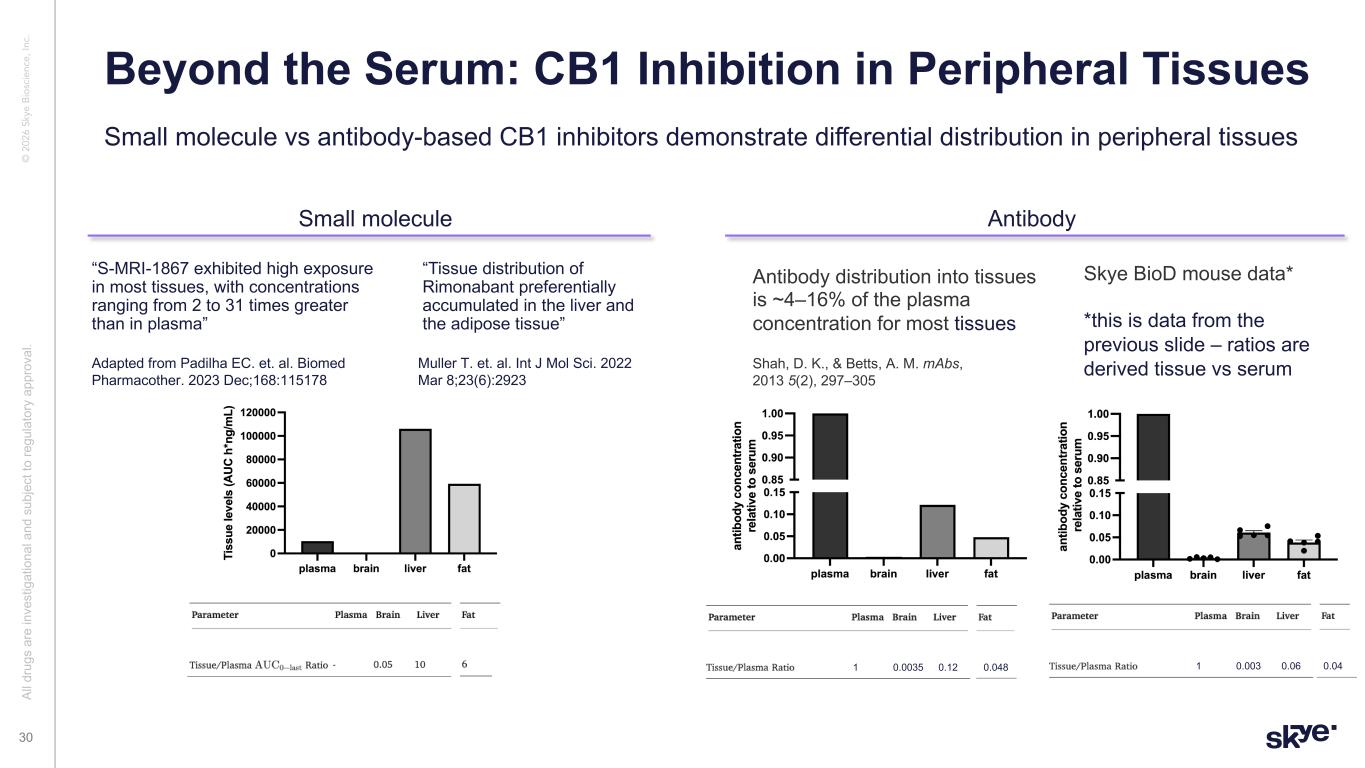
© 2 02 6 Sk ye B io sc ie nc e, In c. Beyond the Serum: CB1 Inhibition in Peripheral Tissues Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 30 “S-MRI-1867 exhibited high exposure in most tissues, with concentrations ranging from 2 to 31 times greater than in plasma” Adapted from Padilha EC. et. al. Biomed Pharmacother. 2023 Dec;168:115178 Muller T. et. al. Int J Mol Sci. 2022 Mar 8;23(6):2923 “Tissue distribution of Rimonabant preferentially accumulated in the liver and the adipose tissue” Small molecule vs antibody-based CB1 inhibitors demonstrate differential distribution in peripheral tissues Small molecule Antibody Antibody distribution into tissues is ~4–16% of the plasma concentration for most tissues Shah, D. K., & Betts, A. M. mAbs, 2013 5(2), 297–305 0.00351 0.12 0.048 0.0031 0.06 0.04 Skye BioD mouse data* *this is data from the previous slide – ratios are derived tissue vs serum
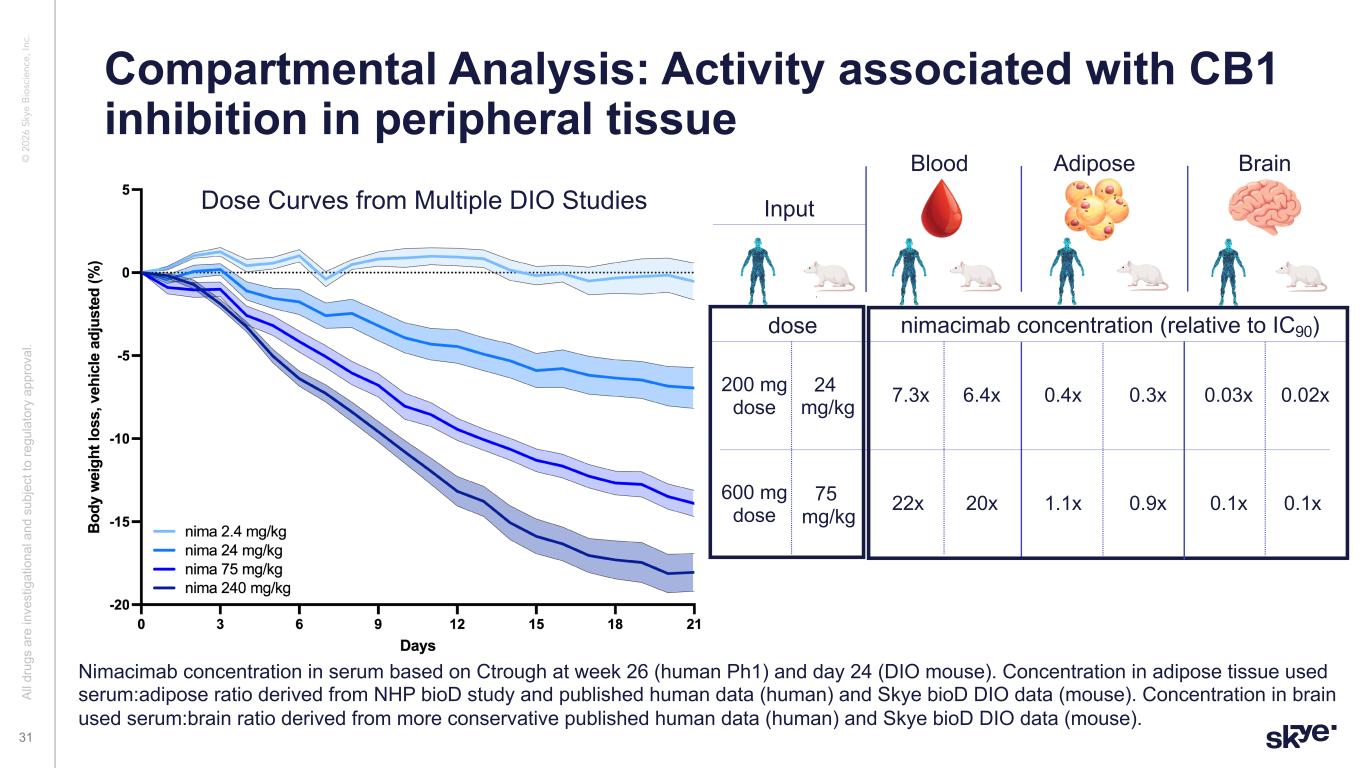
© 2 02 6 Sk ye B io sc ie nc e, In c. Compartmental Analysis: Activity associated with CB1 inhibition in peripheral tissue Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 31 Blood Adipose Brain nimacimab concentration (relative to IC90) 6.4x 20x Nimacimab concentration in serum based on Ctrough at week 26 (human Ph1) and day 24 (DIO mouse). Concentration in adipose tissue used serum:adipose ratio derived from NHP bioD study and published human data (human) and Skye bioD DIO data (mouse). Concentration in brain used serum:brain ratio derived from more conservative published human data (human) and Skye bioD DIO data (mouse). 0.3x 0.02x 0.1x 0.9x 7.3x 0.4x 0.03x 22x 1.1x 0.1x 24 mg/kg 75 mg/kg 200 mg dose 600 mg dose dose Dose Curves from Multiple DIO Studies Input
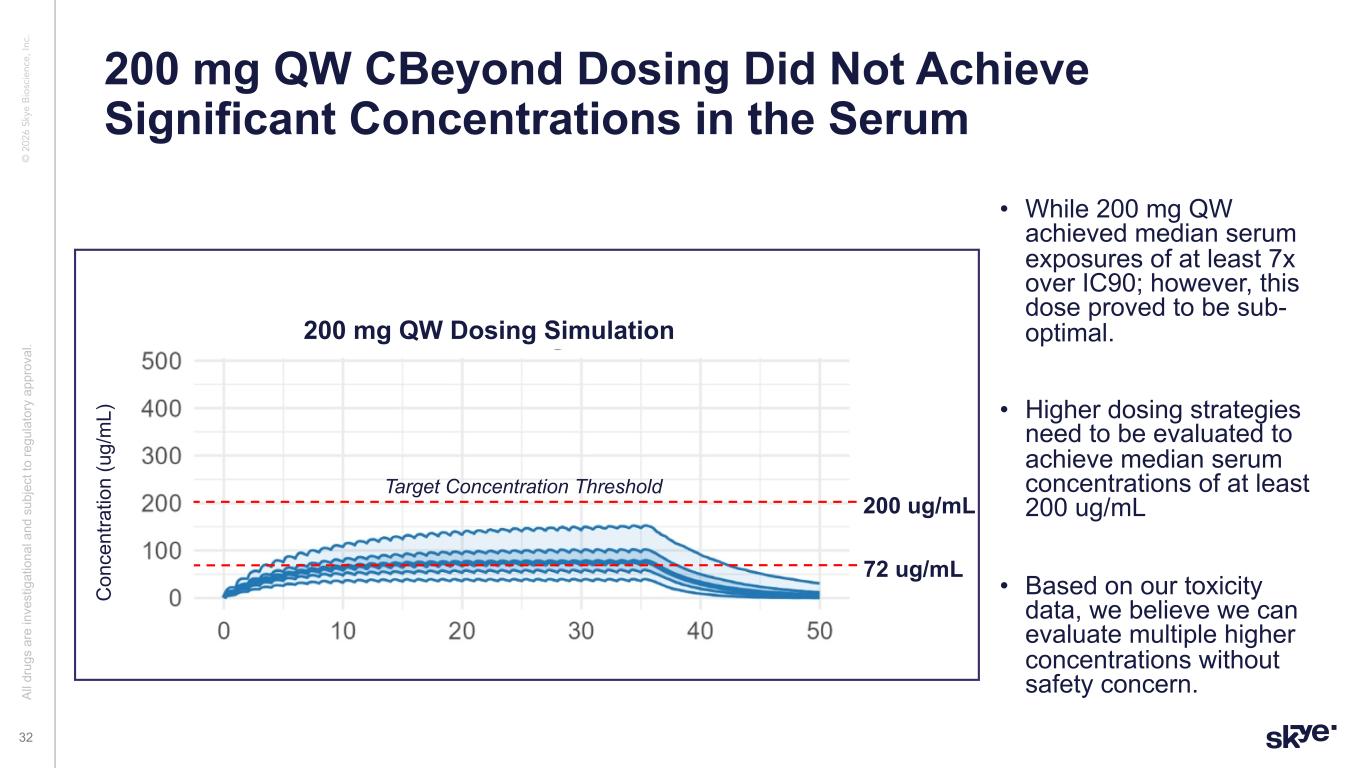
© 2 02 6 Sk ye B io sc ie nc e, In c. • While 200 mg QW achieved median serum exposures of at least 7x over IC90; however, this dose proved to be sub- optimal. • Higher dosing strategies need to be evaluated to achieve median serum concentrations of at least 200 ug/mL • Based on our toxicity data, we believe we can evaluate multiple higher concentrations without safety concern. 200 mg QW CBeyond Dosing Did Not Achieve Significant Concentrations in the Serum Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 32 C on ce nt ra tio n (u g/ m L) 200 mg QW Dosing Simulation 72 ug/mL 200 ug/mL Target Concentration Threshold
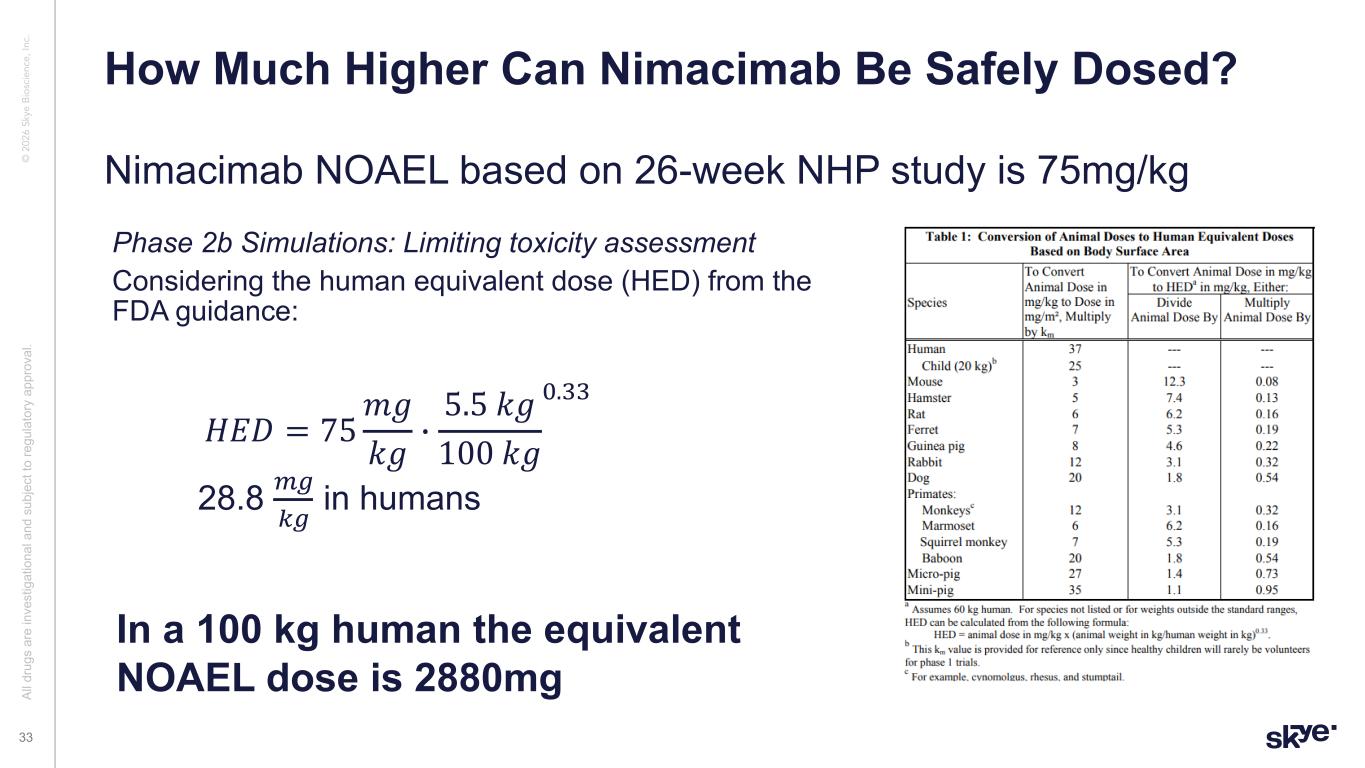
© 2 02 6 Sk ye B io sc ie nc e, In c. Nimacimab NOAEL based on 26-week NHP study is 75mg/kg How Much Higher Can Nimacimab Be Safely Dosed? Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 33 Phase 2b Simulations: Limiting toxicity assessment Considering the human equivalent dose (HED) from the FDA guidance: 𝐻𝐸𝐷 = 75 𝑚𝑔 𝑘𝑔 * 5.5 𝑘𝑔 100 𝑘𝑔 !.## 28.8 $%&% in humans In a 100 kg human the equivalent NOAEL dose is 2880mg

© 2 02 6 Sk ye B io sc ie nc e, In c. • Skye has conducted two non-human primate toxicology studies both establishing 75mg/kg as NOAEL dose. 1. 26-Week Subcutaneous Dosing (N=9) 2. 4-Week IV Dosing (N=10) • Direct concentration comparisons (e.g. Cmax and AUC) in non-human primate PK is highly predictive of human PK due to species similarities (S6 ICH Guidance). • Using these data, we evaluated multiple doses determine safety of potential clinical doses. Modeling NHP Toxicity Study Provides Guidance for Potential Nimacimab Dosing Regimens Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 34 Cmax Weekly AUC 26-Week SubQ Study 2150 ug/mL ~305,000 ug*h/mL 4-Week IV Study 2500 ug/mL ~375,000 ug*h/mL
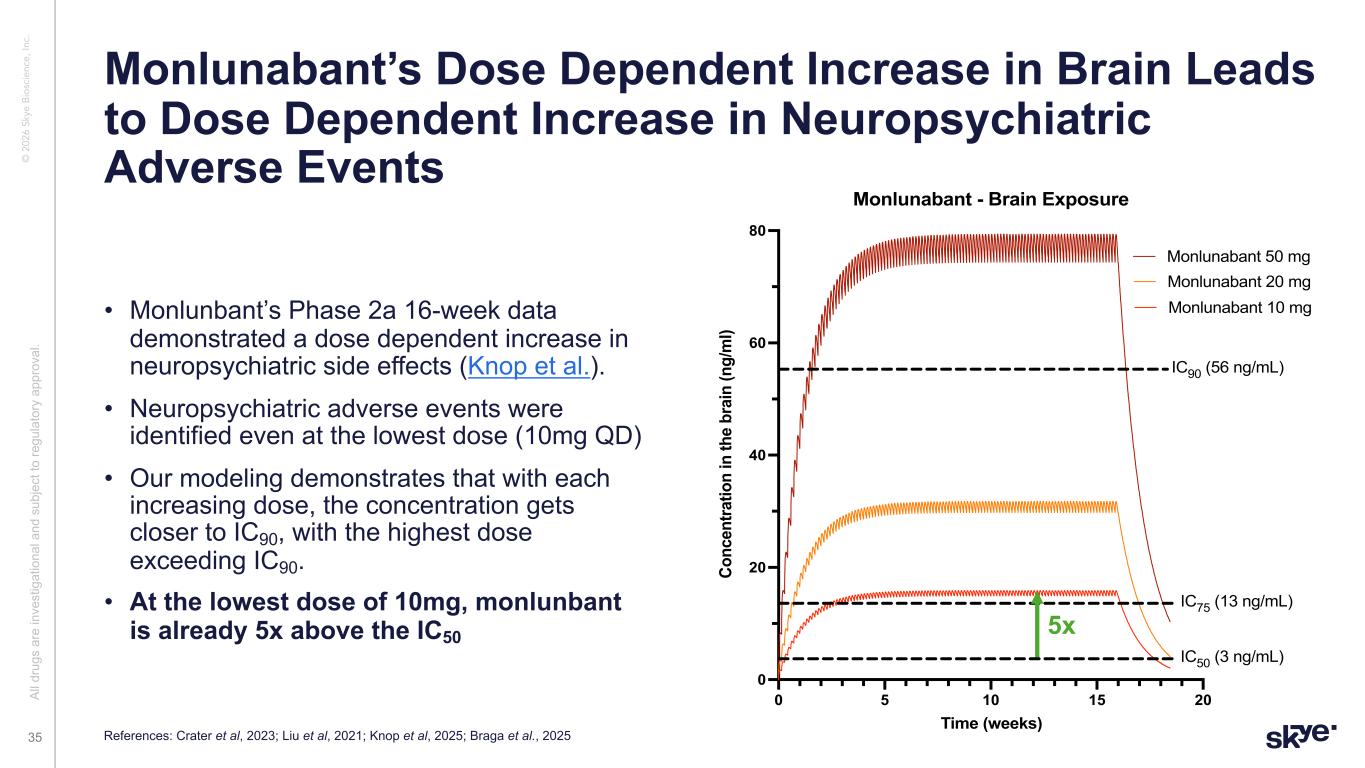
© 2 02 6 Sk ye B io sc ie nc e, In c. Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 35 • Monlunbant’s Phase 2a 16-week data demonstrated a dose dependent increase in neuropsychiatric side effects (Knop et al.). • Neuropsychiatric adverse events were identified even at the lowest dose (10mg QD) • Our modeling demonstrates that with each increasing dose, the concentration gets closer to IC90, with the highest dose exceeding IC90. • At the lowest dose of 10mg, monlunbant is already 5x above the IC50 0 5 10 15 20 0 20 40 60 80 Time (weeks) C on ce nt ra tio n in th e br ai n (n g/ m l) Monlunabant - Brain Exposure Monlunabant 10 mg Monlunabant 20 mg Monlunabant 50 mg IC90 (56 ng/mL) IC50 (3 ng/mL) IC75 (13 ng/mL) Monlunabant’s Dose Dependent Increase in Brain Leads to Dose Dependent Increase in Neuropsychiatric Adverse Events References: Crater et al, 2023; Liu et al, 2021; Knop et al, 2025; Braga et al., 2025 5x
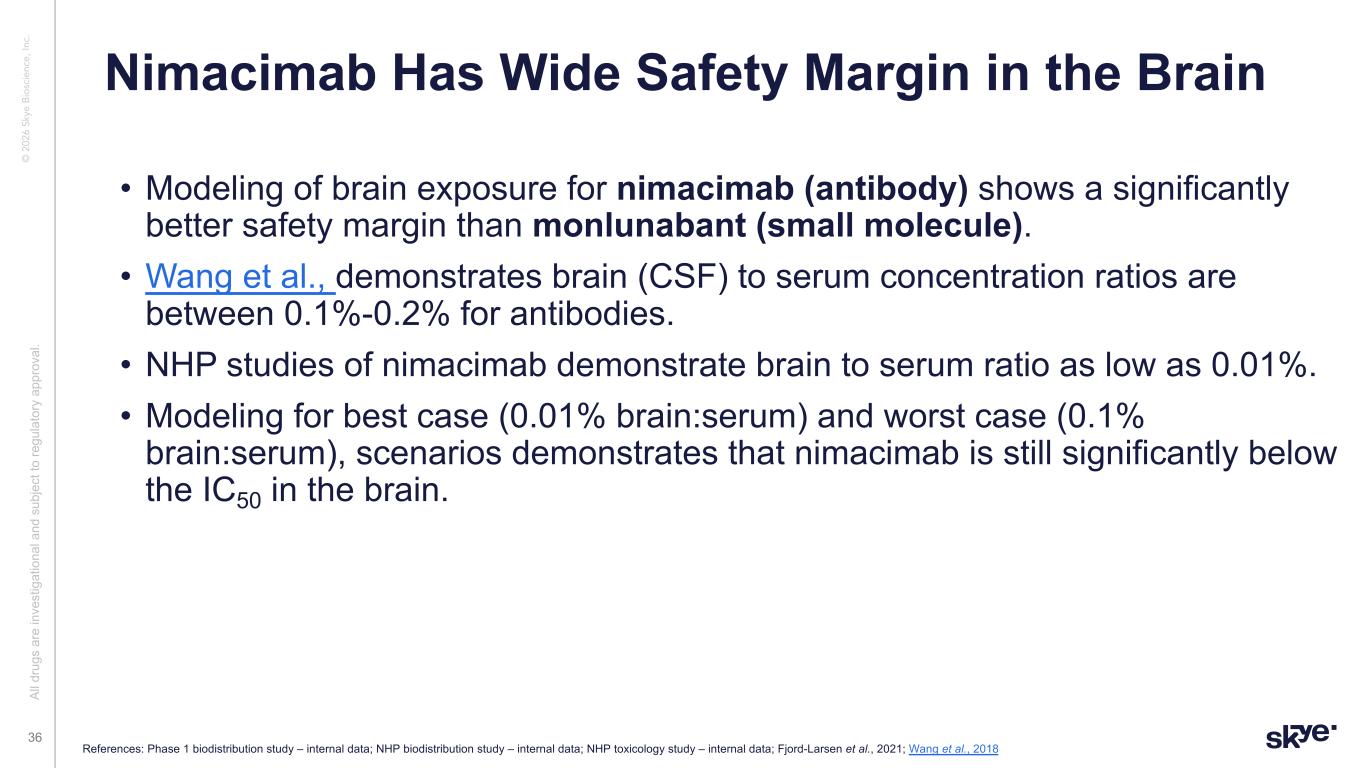
© 2 02 6 Sk ye B io sc ie nc e, In c. Nimacimab Has Wide Safety Margin in the Brain References: Phase 1 biodistribution study – internal data; NHP biodistribution study – internal data; NHP toxicology study – internal data; Fjord-Larsen et al., 2021; Wang et al., 2018 Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 36 • Modeling of brain exposure for nimacimab (antibody) shows a significantly better safety margin than monlunabant (small molecule). • Wang et al., demonstrates brain (CSF) to serum concentration ratios are between 0.1%-0.2% for antibodies. • NHP studies of nimacimab demonstrate brain to serum ratio as low as 0.01%. • Modeling for best case (0.01% brain:serum) and worst case (0.1% brain:serum), scenarios demonstrates that nimacimab is still significantly below the IC50 in the brain.

Clinical Strategy Phase 2 Adaptive Design Dose-Ranging Study 3.0
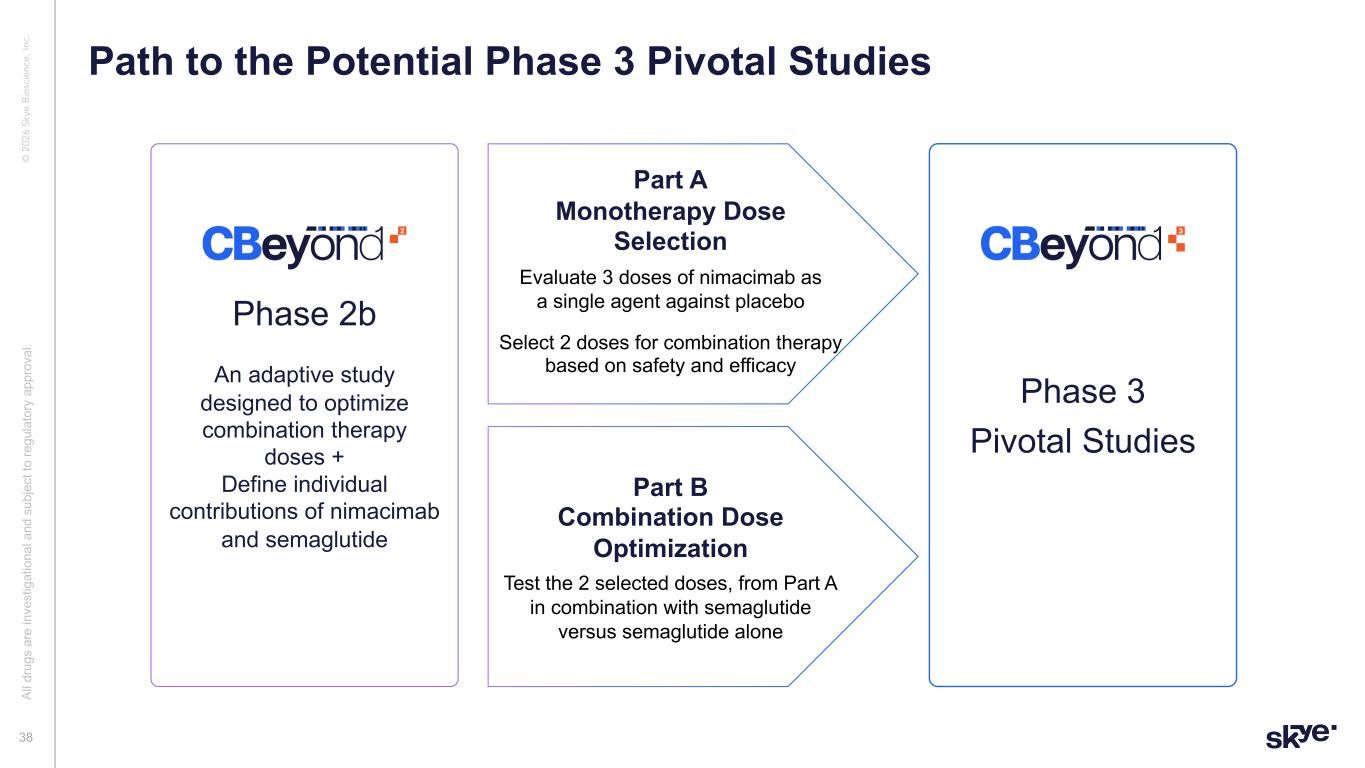
© 2 02 6 Sk ye B io sc ie nc e, In c. Phase 2b An adaptive study designed to optimize combination therapy doses + Define individual contributions of nimacimab and semaglutide Part A Monotherapy Dose Selection Evaluate 3 doses of nimacimab as a single agent against placebo Select 2 doses for combination therapy based on safety and efficacy Part B Combination Dose Optimization Test the 2 selected doses, from Part A in combination with semaglutide versus semaglutide alone Phase 3 Pivotal Studies 38 Path to the Potential Phase 3 Pivotal Studies Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l.
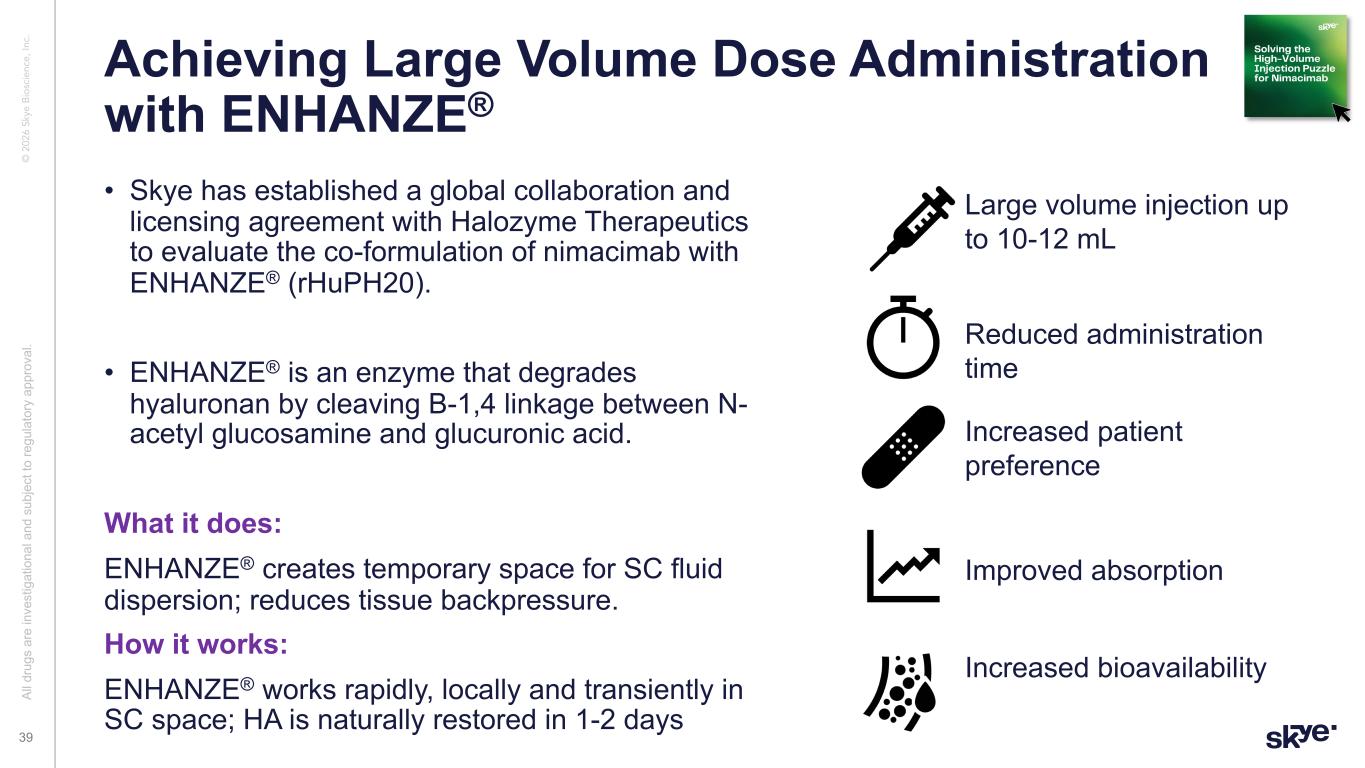
Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. © 2 02 6 Sk ye B io sc ie nc e, In c. Achieving Large Volume Dose Administration with ENHANZE® • Skye has established a global collaboration and licensing agreement with Halozyme Therapeutics to evaluate the co-formulation of nimacimab with ENHANZE® (rHuPH20). • ENHANZE® is an enzyme that degrades hyaluronan by cleaving B-1,4 linkage between N- acetyl glucosamine and glucuronic acid. What it does: ENHANZE® creates temporary space for SC fluid dispersion; reduces tissue backpressure. How it works: ENHANZE® works rapidly, locally and transiently in SC space; HA is naturally restored in 1-2 days 39 Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. Reduced administration time Large volume injection up to 10-12 mL Increased patient preference Improved absorption Increased bioavailability
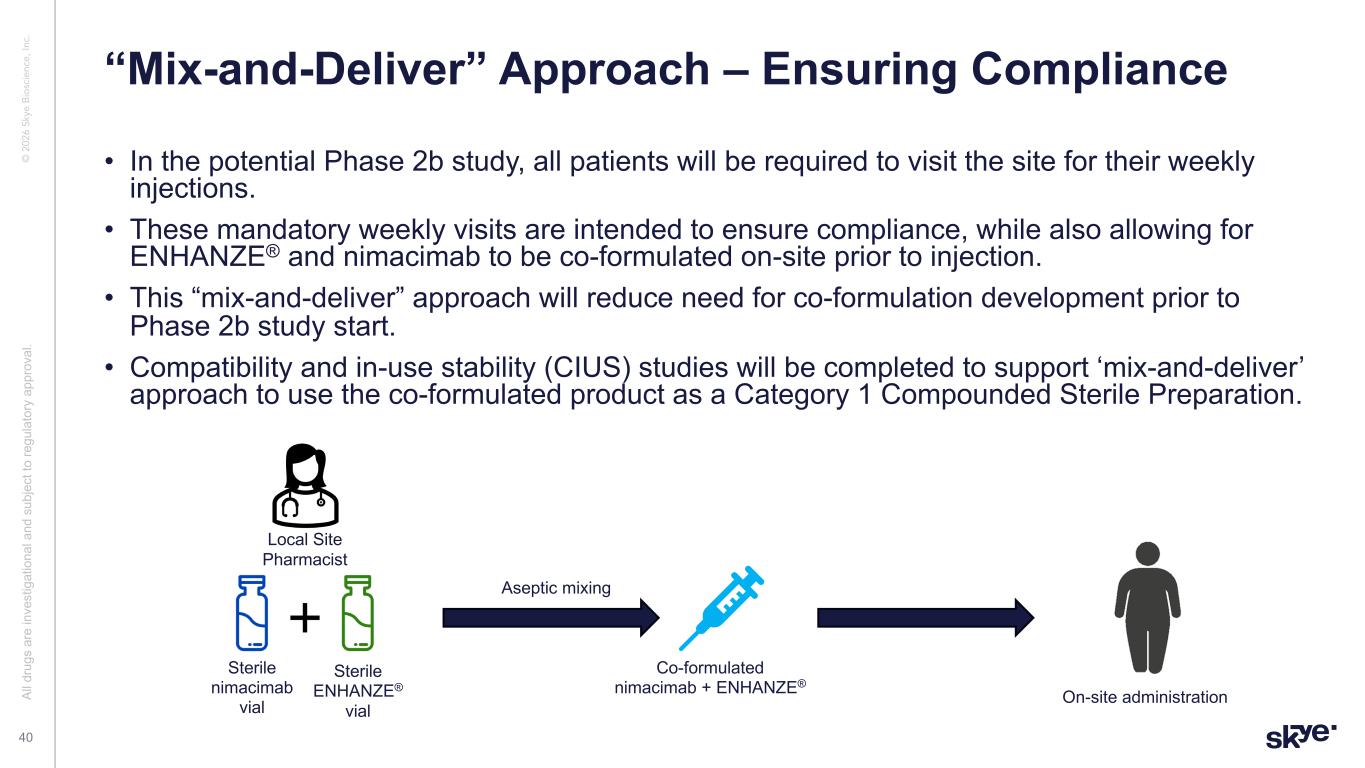
© 2 02 6 Sk ye B io sc ie nc e, In c. • In the potential Phase 2b study, all patients will be required to visit the site for their weekly injections. • These mandatory weekly visits are intended to ensure compliance, while also allowing for ENHANZE® and nimacimab to be co-formulated on-site prior to injection. • This “mix-and-deliver” approach will reduce need for co-formulation development prior to Phase 2b study start. • Compatibility and in-use stability (CIUS) studies will be completed to support ‘mix-and-deliver’ approach to use the co-formulated product as a Category 1 Compounded Sterile Preparation. “Mix-and-Deliver” Approach – Ensuring Compliance Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 40 Sterile nimacimab vial Sterile ENHANZE® vial Local Site Pharmacist Co-formulated nimacimab + ENHANZE® On-site administration Aseptic mixing
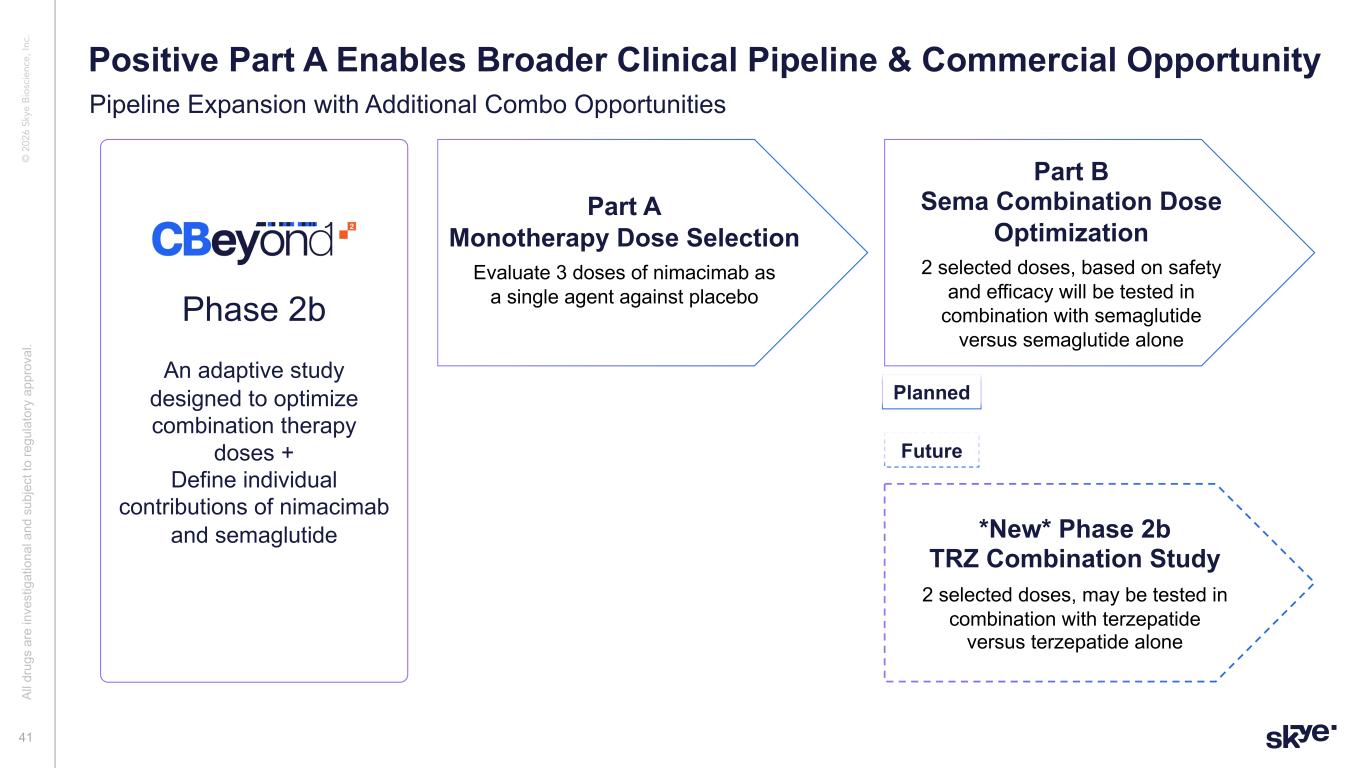
© 2 02 6 Sk ye B io sc ie nc e, In c. Phase 2b An adaptive study designed to optimize combination therapy doses + Define individual contributions of nimacimab and semaglutide Part A Monotherapy Dose Selection Evaluate 3 doses of nimacimab as a single agent against placebo Part B Sema Combination Dose Optimization 2 selected doses, based on safety and efficacy will be tested in combination with semaglutide versus semaglutide alone 41 Positive Part A Enables Broader Clinical Pipeline & Commercial Opportunity Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. *New* Phase 2b TRZ Combination Study 2 selected doses, may be tested in combination with terzepatide versus terzepatide alone Planned Future Pipeline Expansion with Additional Combo Opportunities

Regulatory Strategy Combination Approval, Monotherapy Opportunity 3.1
© 2 02 6 Sk ye B io sc ie nc e, In c. • Phase 2b adaptive design is meant to satisfy the FDA’s requirement to evaluate contribution of parts between nimacimab and semaglutide. • If acceptable, we believe the pivotal Phase 3 design may only require a two-arm study comparing combination of nimacimab + semaglutide versus placebo. • There is precedent for a similar approach as QSYMIA was approved by the FDA on the basis of studies evaluating the combination of phentermine + topiramate versus placebo. Combination Regulatory Strategy Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 43

© 2 02 6 Sk ye B io sc ie nc e, In c. • We believe nimacimab has opportunity to fill gaps in the 2nd line therapy space after patients fail NuSH therapies (i.e. GLP1, GLP1/GIP, or amylin). • If nimacimab alone can demonstrate at least +8% pbo-adjusted weight loss then it could be an ideal 2nd line therapy • Alternatively, nimacimab has the potential as a maintenance therapy, using a similar regulatory path to orlistat (Xenical). • Xenical Label: Monotherapy Opportunities Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 44

Financial Overview and Team 4.0
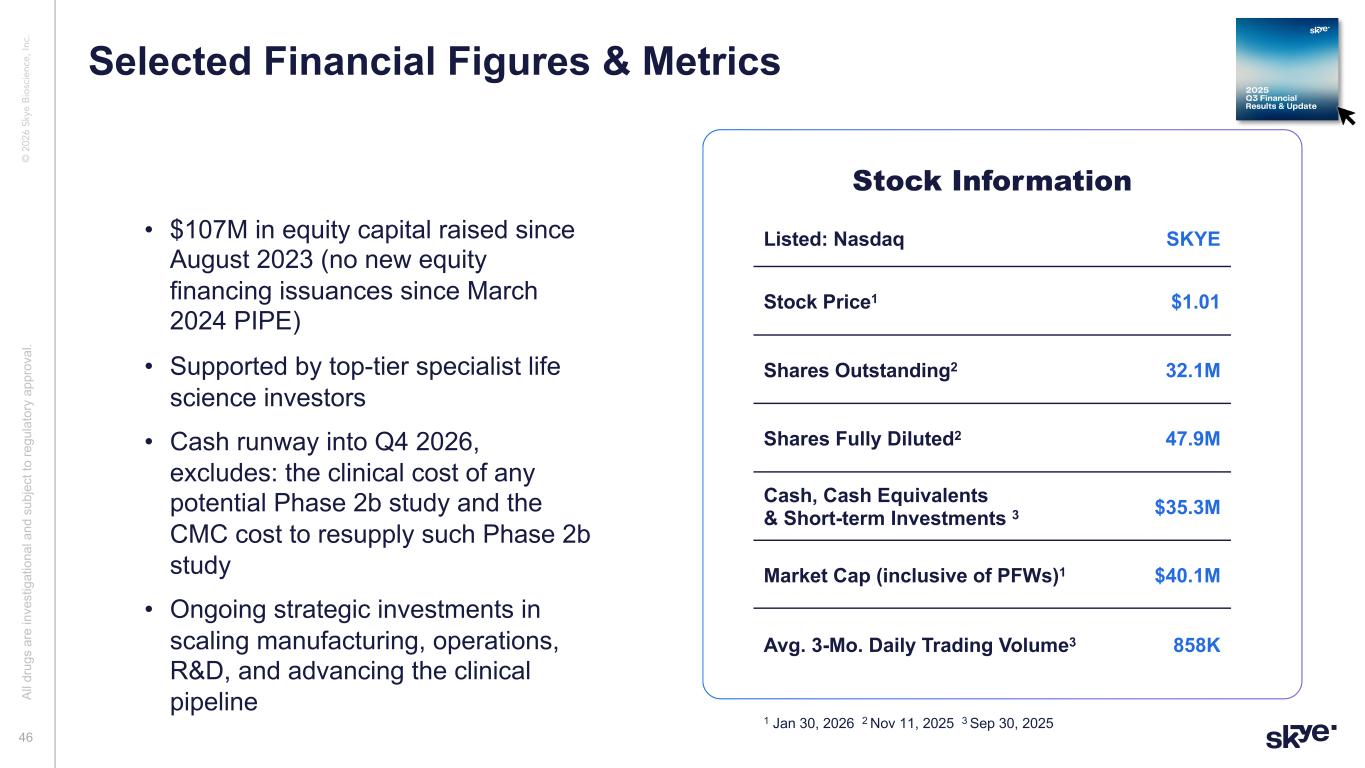
© 2 02 6 Sk ye B io sc ie nc e, In c. • $107M in equity capital raised since August 2023 (no new equity financing issuances since March 2024 PIPE) • Supported by top-tier specialist life science investors • Cash runway into Q4 2026, excludes: the clinical cost of any potential Phase 2b study and the CMC cost to resupply such Phase 2b study • Ongoing strategic investments in scaling manufacturing, operations, R&D, and advancing the clinical pipeline Stock Information Listed: Nasdaq SKYE Stock Price1 $1.01 Shares Outstanding2 32.1M Shares Fully Diluted2 47.9M Cash, Cash Equivalents & Short-term Investments 3 $35.3M Market Cap (inclusive of PFWs)1 $40.1M Avg. 3-Mo. Daily Trading Volume3 858K 1 Jan 30, 2026 2 Nov 11, 2025 3 Sep 30, 2025 Al l d ru gs a re in ve st ig at io na l a nd s ub je ct to re gu la to ry a pp ro va l. 46 Selected Financial Figures & Metrics

Deborah Charych, PhD Co-founder and ex-CTO, RayzeBio Contributed to commercialization of 40+ drugs/diagnostics, led high-value strategic transactions, and co-founded multiple companiesLeadership 47 Executive Management Board of Directors Tu Diep, MSc Chief Operating Officer Chris Twitty, PhD Chief Scientific Officer Kaitlyn Arsenault, CPA Chief Financial Officer Punit Dhillon President & CEO Andy Schwab Managing Partner, 5AM Ventures Paul Grayson Chairman of Skye BOD; Pres./CEO, Radionetics Annalisa Jenkins, MBBS, FRCP Managing Director, Annalisa Jenkins LLC Puneet Arora, MD Chief Medical Officer Karen Smith, MD, PhD, MBA, LLM Global pharma/biotech exec and C-suite advisor Brennen Brodersen, JD General Counsel

THANK YOU 11250 El Camino Real, Suite 100 San Diego, CA 92130 ir@skyebioscience.com Please learn more or contact us at: +1 (858) 410-0266 Spotlight