10-K: Annual report pursuant to Section 13 and 15(d)
Published on March 22, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|||||
For the fiscal year ended December 31 , 2023
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|||||
For the transition period from __________ to __________
Commission File Number: 000-55136
| (Exact name of registrant as specified in its charter) | ||||||||
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|||||||
|
|
||||||||
| (Address of principal executive offices) | (Zip Code) | |||||||
Registrant’s telephone number, including area code: (858 ) 410-0266
Securities registered pursuant to Section 12(b) of the Act:
Title of Class: |
Name of each exchange on which registered: | |||||||
None |
None |
|||||||
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, Par Value $0.001
(Title of Class)
Indicate by check mark if registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
Indicate by check mark if registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ |
Accelerated filer | ☐ |
|||||||||||
☒ |
Smaller reporting company | |||||||||||||
| Emerging growth company | ||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒ Yes ☐ No
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐ Yes ☒ No
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐ Yes ☒ No
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
The aggregate market value of the voting and non-voting common equity held by non-affiliates was approximately $20,402,540 as of June 30, 2023, based upon the closing price of $5.25 per share of the registrant’s common stock on the OTCQB on June 30, 2023, the last business day of the registrant’s most recently completed second fiscal quarter.
As of March 20, 2024, there were 28,062,907 shares of the registrant’s common stock issued and outstanding.
TABLE OF CONTENTS
| PAGE | ||||||||
Item 1A. |
Risk Factors |
|||||||
2
PART I
As used in this report, unless otherwise indicated, the terms “we,” “us,” “our,” “Company” and “Skye Bioscience” refer to Skye Bioscience, Inc., a Nevada corporation, together with its wholly owned subsidiaries, (i) Nemus, a California corporation, (ii) SKYE Bioscience Pty Ltd ("SKYE Bioscience Australia"), an Australian proprietary limited company, (iii) Emerald Health Therapeutics, Inc. ("EHT") a corporation governed by the Business Corporations Act (British Columbia), (iv) Bird Rock Bio Sub, Inc. ("BRB"), a Delaware corporation, (v) Ruiyi Acquisition Corp, a Delaware corporation and (vi) Avalite Sciences, Inc. ("AVI"), a corporation governed by the Business Corporations Act (British Columbia).
FORWARD-LOOKING STATEMENTS
Statements in this Annual Report on Form 10-K contain forward-looking statements that are based on management’s current expectations and assumptions and information currently available to management and are subject to risks and uncertainties. If such risks or uncertainties materialize or such assumptions prove incorrect, our business, operating results, financial condition and stock price could be materially and negatively affected. In some cases, you can identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would” or the negative of these terms or other comparable terminology. Factors that could cause actual results to differ materially from those currently anticipated include those set forth in the section below titled “Risk Factors,” including, without limitation, risks relating to:
•the results of our research and development activities, including uncertainties relating to the discovery of potential product candidates and the preclinical and clinical testing of our product candidates;
•the timing, progress and results of our clinical studies for SBI-100 Ophthalmic Emulsion (SBI-100 OE) and nimacimab and our estimates regarding the market opportunity for SBI-100 OE and nimacimab if approved;
•the early stage of our product candidates presently under development;
•our ability to obtain and, if obtained, maintain regulatory approval of our current product candidates, and any of our other future product candidates, and any related restrictions, limitations, and/or warnings in the label of any approved product candidate;
•our ability to retain or hire key scientific or management personnel;
•our ability to protect our intellectual property rights that are valuable to our business, including patent and other intellectual property rights;
•our dependence on University of Mississippi, third party manufacturers, suppliers, research organizations, testing laboratories and other potential collaborators, including global supply chain disruptions;
•our ability to develop successful sales and marketing capabilities in the future as needed;
•the size and growth of the potential markets for any of our current product candidates, and the rate and degree of market acceptance of any of our current product candidates;
•competition in our industry;
•regulatory developments in the United States and foreign countries; and
•current pending litigation matters, including the Cunning Lawsuit.
We operate in a rapidly changing environment and new risks emerge from time to time. As a result, it is not possible for our management to predict all risks, including the current global economic environment, the impacts of the high inflationary environment, and associated business disruptions such as delayed clinical trials, laboratory resources and supply chain limitations, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. The forward-looking statements included in this report speak only as of the date hereof, and except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this report to conform these statements to actual results or to changes in our expectations.
3
Item 1. Business.
Overview
We are a clinical stage biopharmaceutical company with a mission to pioneer the development of new medicines that unlock the pharmaceutical potential of the endocannabinoid system ("ECS"). Our clinical assets focus on the modulation of cannabinoid receptor 1 ("CB1") to provide novel treatments and alternatives for diseases caused by metabolic disorders, inflammation, fibrosis and neurodegeneration, such as obesity and glaucoma. Our Phase 2 clinical candidates include nimacimab, a negative allosteric modulating antibody that inhibits peripheral CB1 receptors, currently being developed for the treatment of obesity and SBI-100 Ophthalmic Emulsion ("SBI-100 OE"), a CB1 agonist (activator), currently being developed for the treatment of glaucoma and ocular hypertension. Both of these differentiated drug candidates are focused on distinct opportunities with large unmet needs: 1) Obesity - where a patients now need additional treatments that have the ability to preserve muscle tissue and improve metabolic dysfunction either as new monotherapies or in combination which existing treatments, and 2) Glaucoma - where novel drugs with distinct mechanisms are needed, especially those that are safe, well-tolerated and have neuroprotective potential. We have filed and successfully opened an Investigational New Drug ("IND") application with the U.S. Food and Drug Administration ("FDA") for nimacimab in obesity, and we plan to launch a Phase 2 clinical trial to evaluate nimacimab for the treatment of obesity as monotherapy compared against placebo, as well as evaluate the combination of nimacimab and a GLP-1 agonist in Q3 2024, with final data in late 2025. We are also continuing clinical development of SBI-100 OE for glaucoma and ocular hypertension, with the first data read out from our recently completed Phase 2a trial anticipated in Q2 2024.
The Endocannabinoid System
Nimacimab: Peripheral CB1 Inhibitor
The exploration of the CB1 pathway as a therapeutic target has seen a resurgence of scientific interest, particularly for its role in modulating key physiological functions such as appetite regulation, weight loss and related metabolic processes. Interest in CB1 inhibition has grown in part due to the unprecedented efficacy and commercial success but also the limitations of glucagon-like peptide-1 receptor (GLP-1) agonists for the treatment of obesity due to its distinct mechanisms and poor tolerability. While Novo Nordisk's Wegovy® (semaglutide) once-weekly GLP-1 agonist injection for obesity had sales grow 442% to $4.7 billion in 2023, at the same time Novo Nordisk purchased Inversago, a clinical-stage company developing a peripherally-restricted CB1 inverse agonist therapy for weight loss. We believe this is in part because CB1 inhibition holds significant potential in obesity, and other metabolic diseases, as either a stand-alone drug or in combination with Novo Nordisk's GLP-1 agonist therapy, Wegovy®. The history of CB1 inhibition for the treatment of weight loss in obesity is long and well documented. It started with the clinical validation of an approved drug, Accomplia®, along with it's ultimate demise due to serious side effects. This was followed by a period of deep research into better understanding the mechanisms of weight loss, as well as, the corresponding side effects from CB1 inhibition - ultimately leading to a new class of "peripherally restricted" CB1 inhibitors. As a result, we now understand that inhibiting the CB1 receptor in peripheral tissues has the potential to not only cause weight loss, but may also, provide additional benefits such as improving insulin and leptin sensitivity, while specifically targeting fat loss and preserving lean muscle.
In 2006, Accomplia® (rimonabant) was approved for the treatment of obesity by the European Medicines Agency. Rimonabant was a CB1 inverse agonist that inhibited signaling both in the central nervous system and peripherally. Rimonabant consistently induced weight loss of 4-6 kg over 6-12 months vs. placebo, with accompanying improvements in metabolic control and hyperlipidemia (Van Gaal et al. Lancet, 2016, Astrup et al., Lancet, 2007). Subsequent meta-analyses showed that rimonabant increased risk of serious psychiatric adverse events such as anxiety, depression and suicidal ideation. It is believed that rimonabant's accumulation in the brain is the primary reason for the increased risk of serious psychiatric events. However, an increasing body of research suggests that the weight loss benefits of rimonabant may be achieved by targeting CB1 inhibition solely in the periphery and not in the brain, thereby avoiding risk of psychiatric adverse events. Although nimacimab is not in direct competition with GLP-1 agonists, a comparative analysis of rimonabant and semaglutide highlights significant differences in their gastrointestinal (GI) adverse event (AE) profiles. By evaluating rimonabant's GI tolerability and addressing the potential reduced risk of neuropsychiatric AEs with nimacimab—a peripherally acting antibody—nimacimab may emerge as an important next generation mechanisms beyond GLP-1 agonists for healthier, more sustainable weight loss and precision obesity including preserving muscle mass. (Van Gaal et al., Diabetes Care 2008 and Wilding et al., New England J Medicine 2021).
4
Several mouse model studies support the hypothesis that selectively targeting CB1 inhibition exclusively in the periphery results in weight loss as well as other potential benefits. For example, Jenrin Discovery developed JD5037 a CB1 antagonist from ibipinabant with limited brain penetrance. Preclinical studies in the diet-induced obese mouse demonstrated that treatment with 3mg/kg daily of JD5037 over a 28-day period reduced body weight to normal levels. (Tam et al., Cell Metabolism 2012) Mice treated with JD5037 reduced body fat without loss of lean muscle mass. Treated mice experienced restoration of leptin sensitivity by decreasing leptin excretion by fat tissue, which resulted in increased fat loss. When these data are taken together with data from rimonabant, we can conclude that peripheral inhibition of CB1 is sufficient to induce weight loss, while preserving lean muscle mass and avoiding risks associated with inhibiting CB1 in the brain and central nervous system. Additional preclinical evidence also suggests that targeting both GLP-1 and inhibiting CB1 in the periphery with JD-5037 results in restoration of insulin sensitivity that is not seen with GLP-1 agonist therapy alone. (Liu et al., ACS Pharmacol. Transl. Sci. 2021 and Tam et al., Molecular Metabolism 2017)
We are currently developing nimacimab, a patented peripherally-acting negative allosteric modulating CB1 inhibitor, initially for treatment of obesity. As a monoclonal antibody, nimacimab may offer a wider therapeutic window compared to competitive small-molecule peripheral CB1 inhibitors. Animal data generated positive safety data over 26 weeks and showed that, following dosing, nimacimab primarily remained in peripheral tissue outside the brain/central nervous system ("CNS") and the product candidate had a positive PK/PD supportive of once-monthly dosing.
SBI-100 Ophthalmic Emulsion: CB1 Agonist (Activator)
We are also developing a CB1 agonist for treatment of glaucoma and ocular hypertension. Approximately 7 million people in the United States and 60 million people worldwide suffer from glaucoma and ocular hypertension ("OH"), which is caused by slow drainage of the eye and leads to increases in intraocular pressure ("IOP"). Ultimately, increased IOP is believed to be a main cause of optic nerve damage in glaucoma. Currently, only IOP lowering has been proven to be a modifiable risk factor for glaucoma onset and progression, and thus is the only acceptable endpoint for marketing approval by the FDA (De Moraes et al., Sci. Rep. 2023 and Leske et al., Arch Ophthalmol 2003). We believe that the unmet need for treatments in glaucoma is high. Approximately 40% of patients fail first line therapy and approximately 50% of patients require more than one therapy.
SBI-100 OE is a CB1 agonist (activator) and is a patented prodrug of tetrahydrocannabinol ("THC") focused on lowering IOP related to glaucoma and ocular hypertension. Ophthalmology opinion leaders have described the unmet need for a new alternate class of medicine to lower intraocular pressure and ideally provide protection against detrimental neurodegenerative effects on the optic nerve. Third party research has demonstrated the utility of THC to lower intraocular pressure and also indicated its neuroprotective capabilities. In October 2023, Skye announced Phase 1 data indicating this differentiated eyedrop was safe and well tolerated, with a low rate of hyperaemia (red eyes) and no signs of intoxication from exposure to THC. Encouraging signs of reduction in IOP were also observed in the subset of healthy volunteers who had an elevated baseline IOP, indicating the potential utility of SBI-100 OE in reducing IOP in patients with glaucoma or ocular hypertension. We launched a Phase 2a clinical trial of SBI-100 OE in Q4 2023. Enrollment was completed in Q1 2024, with data expected in Q2 2024.
Strategy
Our aim is to be a leader in research and product development focused on the EC system. Our goal is to develop first-in-class products to treat significant unmet medical need in global markets. Our operating strategy emphasizes:
Advancing Our Differentiated CB1 Inhibitor, Nimacimab, with a Focus on Obesity as a Stand Alone and Adjunct Therapy to GLP-1 by:
•Continuing to build on Phase 1 clinical data supporting evidence demonstrating safety and evidence of weight loss through restoring leptin sensitivity and enhancing fat metabolism
•Developing clinical data in Phase 2 supporting our hypothesis that nimacimab may cause weight loss while preserving muscle mass, which is not possible with current GLP-1 and GIP (glucose-dependent insulinotropic peptide) agonist therapies where reduction of muscle mass is a known side effect.
•Showing through clinical data the potential for nimacimab to preserve muscle mass, and induce further reduction in weight loss when used in conjunction with GLP-1 and GIP agonist therapies
•Exploring strategic collaborations for further development and deployment of nimacimab by expanding the clinical trial pipeline
5
Advance Our First of Kind Eye Drop Formulation of CB1 Agonist SBI-100 OE as a New Treatment for Ocular Hypertension and Glaucoma by:
•Continuing our Phase 2a proof-of-concept study to develop evidence supporting our hypothesis that SBI-100 OE will lower IOP in patients with ocular hypertension and glaucoma as it did in healthy patients during our Phase 1 clinical trial
•Initiating a Phase 2b study evaluating SBI-100 OE against a current standard of care
By adhering to this strategy, Skye aims to create medicines that significantly improve the lives of patients around the world. Our focus on the ECS and CB1 modulation reflects our commitment to developing innovative treatments for chronic diseases with large unmet needs characterized by neuropathic, inflammatory, and metabolic processes.
Our Product Candidates
Nimacimab: Peripheral CB1 Inhibitor
Unmet Need & Market Opportunity
Global obesity rates have been rising dramatically, affecting more than one billion people worldwide (approximately 650 million adults). For example, approximately 42.4% of adults living in the United States are obese. This translates to more than 112.5 million American adults with a BMI of >30kg/m2, although it is likely that current rates and patient numbers are higher given obesity prevalence has increased 30.5% since 2000. Obesity is a complex disease characterized by excess and chronic inflammation of adipose tissues, and it results from a chronic energy surplus in which the body’s energy intake exceeds its energy expenditure. Other stressors and environmental factors contribute to this chronic imbalance. Today, obesity is the fifth-leading risk factor cited by the World Health Organization ("WHO") for contributing as a primary cause of death globally. Current estimates by the World Obesity Atlas suggest that over half the global population will be overweight or obese by 2035, compared to 38% in 2020.
Early-generation therapies for obesity offered only modest help in weight reduction, with some drugs being removed from the market due to safety concerns. More recently, a new class of drugs have proven very effective at achieving weight loss. These incretin mimetics, including GLP-1 agonists such as semaglutide and GLP-1/GIP combinations such as tirzepatide, have changed the paradigm for anti-obesity treatments. Products derived from these compounds suppress appetite and induce satiety, reduce gastric emptying, and thus reduce calorie intake, achieving weight-loss of up to 20% within one year. However, these drugs come with some potentially significant gastrointestinal side effects that may arise, including diarrhea, nausea, abdominal pain and bloating, and in some cases patients may suffer from a condition known as gastroparesis, or paralyzed stomach. Additional side effects, although infrequent, include instances of acute pancreatitis, gallbladder disease, hypoglycemia, acute kidney injury and diabetic retinopathy (in diabetics). Beyond these potential side effects, it has been observed that muscle mass loss accounts for up to 40% of the weight lost in patients using these drugs, which is higher than the typical loss of approximately 25% muscle mass loss in normal weight loss settings. As a result of these side effects many patients are intolerant to these incretin-mimetic drugs. In most cases, even when therapy is tolerated, patients are unable to get to a healthy weight. When taken off therapy, patients can gain a majority of their weight back within the first year. Nevertheless, the market for this class of drugs is projected to grow to $20 billion in sales by 2030. Despite this unprecedented efficacy and commercial success, we believe there remains room for improvement over these new therapies.
Potential of the Body's Endocannabinoid System for Pharmaceutical Drug Development in the Treatment of Obesity
The cloning of CB1, along with the discovery of its endogenous ligands, the endocannabinoids ("ECs"), anandamide ("AEA") and arachidonoylglycerol ("2- AG"), has illuminated some of the biological intricacies and mechanisms involved in fat storage and development of obesity. ECs, CB1, and closely related cannabinoid type-2 receptors constitute the endocannabinoid system ("ECS"). The ECS primarily functions to regulate energy storage and balance within the body, facilitated by CB1's activity in the brain and peripheral tissues. The ECS helps maintain many physiological processes by enabling different cellular functions, all with a role in achieving a global homeostasis despite fluctuations in the external environment. ECs are produced in the body, where their synthesis and degradation is regulated by different enzymes. These endocannabinoids act as agonists that can bind to and signal via specific endocannabinoid receptors expressed on various cell types and tissues.
6
The CB1 receptor is one of the most abundant ECS receptors in the body. It is predominantly found in the CNS, where its role is associated with motor control, cognition, and emotional processing, and including modulation of processes of the eye. CB1 receptors are also found in peripheral tissues, or outside of the CNS, such as the liver, kidney, adipose tissue (fat cells), pancreas, and the gastrointestinal tract. One notable role of CB1 receptors in peripheral tissues is their involvement in metabolic regulation. In adipose tissue, activation of CB1 receptors has been linked to the promotion of fat storage, suggesting their role in the balance of energy storage and utilization. In the kidney, CB1 signaling helps modulate blood flow vital for the filtration of waste products and excess fluids, a process essential for maintaining proper electrolyte balance and blood pressure. CB1 in the liver is involved in the regulation of lipid metabolism and glucose homeostasis. Activation of CB1 receptors in the gastrointestinal tract can modulate the release of neurotransmitters and hormones that influence appetite, gastric motility, and nutrient absorption.
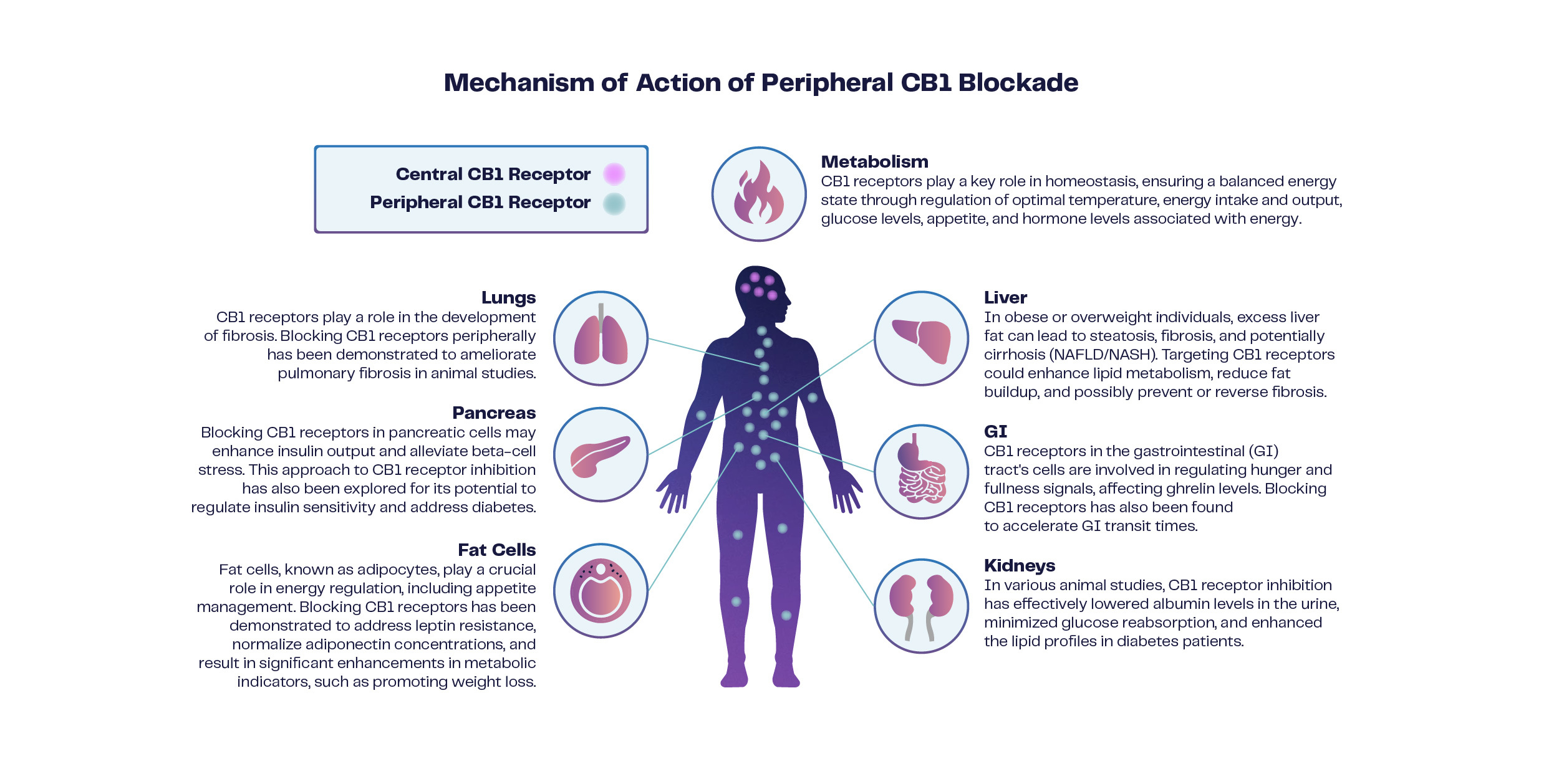
The distribution and function of the CB1 axis provides a strong rationale to target this critical physiological system as a therapeutic to treat different pathological states. Moreover, dysregulation of the CB1 axis in peripheral tissues has been associated with metabolic disorders such as obesity and kidney disease. Operating as a G-protein coupled receptor, CB1 typically associates with inhibitory Gi/o proteins to reduce cellular activity by inhibiting adenylyl cyclase and cAMP activity. Additionally, CB1 interaction with ß-arrestin can initiate a distinct signaling cascade, the full implications of which are not yet completely understood. This ß-arrestin pathway is known to affect receptor sensitivity and expression levels. Recent research highlights that inhibiting CB1 outside the brain can influence leptin sensitivity and adipocyte signaling, directly impacting fat cell physiology. Activation of CB1 promotes fat accumulation and disrupts mitochondrial function in obesity models, whereas inhibiting CB1 enhances mitochondrial biogenesis. This metabolic adjustment results in weight loss primarily through heightened energy expenditure, increased lipolysis, and fatty acid oxidation processes, particularly in brown adipose tissue, which serves as an energy source, contrasting white adipose tissue's role in long-term fat storage.
It has been previously demonstrated that inhibiting the CB1 receptor can significantly reduce weight in obese patients. In 2006, Sanofi developed a CB1 inverse agonist called rimonabant, which demonstrated 8-10% weight loss after one year. Despite being approved by the European Medicines Agency, the drug was soon taken off the market because it was shown to result in a higher risk of adverse psychiatric side effects. As a result, rimonabant was taken off the market in Europe and Sanofi, along with other large pharmaceutical companies like Merck and Pfizer, stopped all development of CB1 inhibitors.
Since the demise of rimonabant, a significant amount of research was conducted to better understand the CB1 blockade and why drugs like rimonabant failed. Ultimately it was determined that rimonabant readily entered the brain, resulting in significant psychiatric side effects. However, it was also discovered that blockade of CB1 receptors outside of the brain (i.e. peripheral CB1 receptors), could also result in meaningful weight loss and also improvement in other metabolic parameters. These studies have led to the development of "peripherally restricted" CB1 inhibitors that have significantly less penetration into the brain, resulting in a potentially safer drug while maintaining potentially similar weight loss characteristics. Moreover, researchers have shown that peripheral blockade of the CB1 receptors can also result in weight loss with less muscle mass loss (i.e. muscle wasting), while also improving metabolic parameters important in changing the course of the disease in obesity, such as improving insulin sensitivity.
7
We believe incretin mimetics, like GLP-1 agonists, have established themselves as a potential foundation for the therapeutic treatment of weight loss. However, with their potentially challenging safety profile and marked incidence of muscle wasting associated with weight loss, we believe it is imperative to develop new drugs that can be complimentary to GLP-1 agonists, such as in combination with, as a follow-on to standard-of-care GLP-1 therapies, or even as an alternative therapy for obese patients who can not tolerate the side effects of GLP-1 agonists. Peripheral CB1 inhibition represents an important new mechanism with potential to help reduce these challenges and create improved therapeutic outcomes.
Technology
In 2023, Skye acquired BRB along with its lead asset, nimacimab, a humanized IgG4 negative allosteric modulating (NAM) antibody that specifically binds to CB1 receptor and does not cross-react with other GPCRs including CB2. CB1 binding characteristics including specificity and affinity have been confirmed with both ELISA and flow cytometry-based assays. Assessing functional inhibition including demonstration of noncompetitive binding CB1 has been confirmed with both b-arrestin-based and GPCR-based endpoints. This functional and specific CB1 inhibiting antibody has also been characterized in multiple biodistribution assays, which collectively demonstrated a high degree of peripheral restriction with minimal detection in the CNS and brain in early time points as well as no accumulation in the brain over 28 days, despite nimacimab's 18-20 day half-life. This lack of blood-brain barrier (BBB) penetration, while not uncommon for a larger biologic such as an antibody therapeutic, is an important feature that helps underpin nimacimab's excellent safety profile.
While different strategies have been developed to limit CB1 signaling, nimacimab's negative allosteric approach is differentiated relative to an inverse agonist inhibitor in a few notable ways. A fundamental functional difference relates to the ability of nimacimab to inhibit in a non-competitive fashion. Since nimacimab limits the receptor activity by binding away (allosterically) from the agonist (endocannabinoid) receptor binding pocket, it is able to inhibit the CB1 signal independently of receptor engagement. In contrast, an inverse agonist competes with the endocannabinoid for receptor binding and thus inhibition of CB1 signaling.
While this distinction may not be as impactful in healthier patients with minimal pathology, this mechanistic distinction becomes more relevant in patients with notable disease progression where the CB1 axis (both receptor but importantly endocannabinoid density) can be significantly overactive in local tissues, enabling competition with an inverse agonist for receptor occupancy. This difference can be exaggerated when the bioavailability of an inverse agonist is limited, which highlights a second key distinction between nimacimab, an antibody therapeutic, and inverse agonists, which are currently all small molecule therapeutics.
Small molecule-based CB1 inverse agonists have been modified to discourage distribution in the CNS and brain by increasing the polarity of surface residues, which also impacts membranous trafficking and bioavailability. While this altered small molecule yields a notable improvement in CNS distribution relative to non-biased small molecules such as rimonabant, its presence and significant CB1 occupancy in the brain has still been noted in a chronic preclinical setting. Coupled with an impact on bioavailability, this may limit the therapeutic index and thus its relative density in local tissues.
In contrast, nimacimab has an excellent clinical safety profile with a large NOAEL (no observed adverse effect level) and an 18-20 day half-life which collectively supports a favorable pharmacologic profile capable of safely inhibiting CB1 signaling in relevant disease settings.
Nonclinical Data
Nonclinical studies were designed to characterize various pharmacodynamic aspects of nimacimab, including specificity and in vitro affinity binding, functional cell-based inhibition, as well as other mechanistic details. Key findings from these studies demonstrated that nimacimab is selective and does not bind to other GPCR targets while binding with low nM affinity to human CB1. The antibody was able to block CB1 activation by the natural endocannabinoid ligands AEA and 2-AG and demonstrated dose-dependent antagonist activity measured by both cAMP and b-arrestin signaling pathways. Pre-treatment with nimacimab blocked CB1 agonist-induced CB1 internalization and nimacimab did not induce internalization in the absence of agonist. Additionally, nimacimab had no effect on antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC), suggesting that nimacimab's mechanism of action is focused on blocking CB1 signaling and not any direct immune-mediated effects. Lastly, antibody blockade of CB1 on matured adipocytes from obese subjects significantly increased adiponectin production compared to vehicle control. These findings are of importance as adiponectin, an adipokine secreted by adipocytes, play a critical role in regulating glucose levels, lipid metabolism, and insulin sensitivity, which collectively support positive metabolomic regulation.
8
Clinical Data
In a Phase 1b entitled "Safety, Tolerability and Pharmacokinetics of Nimacimab after Repeat Dosing in Subjects with Non-Alcoholic Fatty Liver Disease (NAFLD)," the purpose was to evaluate the safety and tolerabilty of multiple doses of nimacimab after four weeks of dosing in subjects with NAFLD. Secondary objectives included determination of pharmacokinetics of nimacimab for multiple doses, and to determine levels of anti-drug antibodies (ADA) after dosing with nimacimab. In Part A of this study, 24 healthy volunteers were randomized to receive a single dose of either placebo or single ascending doses of nimacimab (0.6, 1.2 or 2.5 mg/kg). In Part B, 82 patients with pre-diabetes or diabetes and NAFLD were randomized to receive either placebo or multiple escalating doses of nimacimab (0.6, 1.2 or 2.5 mg/kg) once a week for four weeks.
There were no deaths, serious adverse events (SAEs) or treatment-emergent adverse events (TEAEs) that lead to discontinuation. All TEAEs were graded as mild to moderate in intensity except for 1 severe TEAE (dizziness) in the nimacimab 0.6 mg/kg dose group determined to have not been related to study drug. The majority of TEAEs were not related to study drug and there was no apparent relationship between the dose level and the type, severity, or incidence of the TEAEs.
For all subjects at all doses, concentrations of nimacimab were quantifiable in serum by 0.5 hours after the first dose and remained quantifiable in most subjects through the last time point (Day 67). Exposure to nimacimab as measured by AUC and Cmax increased with increasing doses of nimacimab. At all dose levels, median Tmax ranged from 0.5 to 2 hours and mean t1/2 ranged from 18 to 22 days.
Immunogenicity was assessed in all subjects throughout the study. Only two subjects had consistently elevated titers over multiple time points of ADA. These data suggest that nimacimab has overall low immunogenicity.
Decreases in mean alanine transaminase (ALT) and aspartate aminotransferase (AST) were observed during the study in the active treatment groups, but not in the placebo group. Numeric decreases in ELF score and statistically significant reduction in hyaluronic acid (HA) was observed in the 1.2 mg/kg dose group compared to placebo (p=0.02). Assessment of serum lipids indicated a dose-dependent trend towards reduction of low-density lipoprotein cholesterol (LDL-c) starting from Day 29; on Day 67 there was a mean 8.9 mg/dL decrease in LDL-c in the 2.5 mg/kg dose group compared with a mean 8.3 mg/dL increase in the placebo group (p=0.0073). No effect was observed in total cholesterol, high-density lipoprotein cholesterol (HDL-c), and triglycerides. No significant treatment effect was observed on liver fat percentage, DNL, inflammatory biomarkers and OGTT test.
Development Plan
IND-enabling studies of nimacimab in non-human primates demonstrated a strong safety profile with a NOAEL of 75 mg/kg. Our Phase 2 study in obesity is designed to evaluate nimacimab's weight loss potential either as a single agent or in combination with a GLP-1 agonist like semaglutide. We believe the complementary mechanism of action of CB1 inhibition with nimacimab in combination with a GLP-1 agonist has the potential to provide more meaningful weight loss, and potentially more durable weight loss, than a GLP-1 agonist alone.
Our Phase 2 clinical trial design will treat non-diabetic individuals with obesity (BMI≥30) or those overweight (BMI≥27) with at least one weight-related health issue. Participants will be treated with either nimacaimb, a GLP-1 agonist such as semaglutide, a combination of nimacimab plus a GLP-1 agonist, or placebo. This 26-week study will be followed by a 12-week observation period. The main goal is to measure the percentage of weight loss by week 26, with secondary goals assessing changes in waist size, body composition, fasting triglyceride levels, cholesterol, and A1c. The study will help us evaluate the effectiveness of nimacimab alone versus placebo, as well as compare the difference between nimcacimab and semaglutide and the potential enhanced effects of their combination. A key focus is the impact on body composition across different groups, especially since nimacimab is expected to better preserve muscle mass by promoting fat browning, a benefit observed in preclinical studies where CB1 inhibition led to significant weight reduction without muscle loss over 28 days. We plan to finalize the trial design and start the Phase 2 study in approximately mid-2024.
SBI-100 Ophthalmic Emulsion: CB1 Agonist (Activator)
Unmet Need & Market Opportunity
Glaucoma is characterized by progressive ocular neuropathy associated with the initiation of apoptosis of retinal ganglion cells ("RGCs") in the optic nerve, which can progress and cause irreversible loss of vision. While the pathology of glaucoma is in the back of the eye, a key driver of this disease is often found in the front of the eye. Specifically, elevated intraocular pressure ("IOP") resulting from dysregulated outflow, and to a lesser extent production of aqueous humor ("AH") in the anterior/posterior chambers in the front of the eye, readily promotes damage to RGC axons through vascular ischemia and physical crush injury as the elevated ocular pressure compresses these critical cells. IOP is currently the only modifiable risk factor and lowering IOP results in reduced risk of progression of damage to the optic nerve. Moreover, IOP has been identified as an important risk factor in the pathogenesis of this disease.
9
To reduce IOP, the current classes of glaucoma therapeutics target pathways that shift the balance of production or outflow of AH. This therapeutic landscape is made up of five drug classes focused on lowering IOP toward a target level whereby the rate of disease progression will be slowed sufficiently to avoid functional impairment from the disease. Prostaglandin analogues and β-blockers are the first-line therapies and the most commonly used medications for the management of glaucoma. There are other therapeutic agents less commonly used to reduce IOP, like cholinergic agonists, alpha agonists and ROCK inhibitors. While these treatments can be effective in some patients, side effects and lack of durable responses often lead to many patients progressing through multiple therapies in an effort to control IOP. Current treatments are legacy classes of drugs thus there is an unmet need for combinations and innovation in this therapeutic landscape. This unmet need is a potential opportunity for Skye's SBI-100 OE to have a positive impact in patients with glaucoma.
Technology
Cannabinoid receptors are highly concentrated in the eye, especially in the anterior compartment that helps regulate IOP, and in the posterior compartment in the area of the retina and optic nerve. Activation of CB1 has previously been shown to lower IOP in both animal and human studies. SBI-100 OE represents a novel treatment option for glaucoma. The active pharmaceutical ingredient ("API"), SBI-100, is synthetically created using rational drug design and biochemical engineering to increase the hydrophilic properties of Δ9-tetrahydrocannabinol. This increased solubility coupled with a proprietary formulation to increase stability and residency time on the ocular surface allows for increased ocular tissue penetration. Once inside the eye, abundant esterases release the prodrug moiety, allowing for an active THC molecule to bind to abundant CB1 receptors throughout key ocular tissues such as the trabecular meshwork and ciliary body. Engagement of these receptors has been demonstrated to promote AH outflow via the trabecular meshwork as well as limit production via the ciliary body. Importantly, the active component of SBI-100 OE has been shown to have neuroprotective effects in both in vitro and in vivo studies. Since SBI-100 OE has been detected in retinal tissues in biodistribution studies, we are investigating if SBI-100 OE may additionally demonstrate a neuroprotective aspect in its treatment of glaucoma, providing further differentiation from the current therapies on the market.
We licensed SBI-100, the active pharmaceutical ingredient in SBI-100 OE, from the University of Mississippi ("UM"). SBI-100 OE is initially being developed to treat glaucoma and ocular hypertension. SBI-100 is Δ9-tetrahydrocannabinol-valine-hemisuccinate (Δ9-THCVHS), a synthetically manufactured amide ester prodrug of Δ9-tetrahydrocannabinol molecule formulated in a proprietary nanoemulsion formulation. In contrast to the parental Δ9-tetrahydrocannabinol molecule, which has very poor bioavailability, this novel synthetic molecule has been shown to penetrate into ocular tissue, where the prodrug moiety is quickly removed, allowing for the delivery of measurable amounts of active drug (Δ9-tetrahydrocannabinol) to the cornea, AH, iris-ciliary body, and retina choroid (RC), permitting interaction with cannabinoid receptors involved in regulating IOP.
Preclinical Data
In 2019, UM completed experiments showing that SBI-100 was statistically superior in lowering IOP compared to the prostaglandin-based therapy latanoprost, the current standard-of-care for treating glaucoma. Statistical significance was reached across multiple time points during a seven-day course of dosing using a validated rabbit normotensive ocular model and SBI-100 exerted pharmacologic activity consistent with once-daily to twice-daily dosing. Skye subsequently developed a proprietary nanoemulsion formulation to optimize the amount of SBI-100 that can be delivered to the eye in a single drop while also improving the duration of activity. Importantly, this formulation can be sterilized by filtration without impacting the attributes of SBI-100. This final formulation of the API, known as SBI-100 Ophthalmic Emulsion, significantly reduced IOP compared to other commercially available ophthalmic solutions and is now being evaluated in clinical trials.
We evaluated the mechanism of action and IOP-lowering ability of the active moiety of SBI-100 when administered into an ex vivo model of a 3D-human trabecular meshwork using both healthy and glaucomatous-induced tissues. The trabecular meshwork plays a key role in removing a vital functional liquid in the eye, called aqueous humor, in order to maintain a healthy balance of pressure in the eye (an imbalance can lead to a detrimental increase in IOP). This study validated the mechanism of action of SBI-100 in lowering IOP, a defining disease process of hypertensive glaucoma. Moreover, biomarkers associated with inflammation and fibrosis in both normal tissue and tissues affected by glaucoma were significantly decreased, pointing to anti-inflammatory and anti-fibrotic activities often associated with cannabinoids in other disease states. Data also revealed that biomarkers associated with neovascularization, a disease process of new blood vessel formation that can damage the retina in a variety of ocular diseases, was also inhibited by the active moiety, prompting further study for the utility of this drug in diseases of the retina.
10
Clinical Data
We commenced dosing of the Phase 1 clinical study in December 2022 and in November 2023 we reported that our Phase 1 data demonstrated that SBI-100 OE was safe and well-tolerated. Importantly, we determined that there was minimal systemic exposure of the active metabolite of SBI-100 OE, THC, thus resulting in little to no side effects related to THC intoxication. Moreover, it was determined that after multiple days of dosing we saw minimal hyperaemia (i.e. redness of the eyes) following administration of SBI-100 OE.
In December 2022, we obtained FDA clearance of our Investigational New Drug application to conduct clinical studies in the US and in January 2023, our Phase 2 clinical trial protocol received study level approval from a central institutional review board (“IRB”). In February 2024, we announced that we completed enrollment of our Phase 2a clinical study for glaucoma and ocular hypertension. Due to the early completion of enrollment of our Phase 2a study, topline data will be available in Q2 2024.
Development Plan
As a novel agent for the potential treatment of glaucoma and ocular hypertension, SBI-100 OE may represent a new treatment opportunity for physicians and patients and we are leading the field in this area of research.
We are currently advancing a Phase 2a trial of SBI-100 targeting primary open-angle glaucoma (POAG) and OH. This study enrolled 56 patients with POAG and OH, presenting intraocular pressures (IOP) between 21-34mmHg, and with no history of surgical interventions. Participants were randomized to receive either 0.5% SBI-100, 1% SBI-100, or a placebo, administered twice daily over a 14-day period. The study's main goal is to assess changes in diurnal IOP compared to placebo. Secondary objectives are to evaluate safety, tolerability, any psychotropic effects, changes in diurnal IOP from the study's start, and to explore potential biomarkers.
Following the data release of our proof-of-concept Phase 2a study, we expect to initiate a Phase 2b study evaluating SBI-100 OE against a current standard of care such as timolol. Successful completion of this Phase 2b study by the end of 2025 may present an opportunity to hold an end-of-Phase 2 meeting with the US FDA to discuss our plan for Phase 3 studies for marketing authorization in glaucoma.
Competition
Our industry is characterized by rapidly advancing technologies, intense competition, rapid pace of new innovation, and a strong emphasis on proprietary products and defense of intellectual property. We face competition from pharmaceutical companies, including generic drug companies, biotechnology companies, drug delivery companies, and academic and research institutions, among others.
Competitors to nimacimab that are targeting peripheral inhibition of CB1 for the treatment of obesity and metabolic conditions include Inversago (acquired by Novo Nordisk in 2023) and Corbus Pharmaceuticals. We are not aware of any competitor attempting to inhibit CB1 in the periphery via a monoclonal antibody. There is also significant interest in and competitive activity targeting obesity and metabolic disorders from companies including Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Zealand Pharma, Pfizer, AstraZeneca, Regeneron, Carmot Therapeutics (acquired by Roche), Amgen, Viking Therapeutics, Structure Therapeutics, Versanis (acquired by Eli Lilly), Biohaven, Keros Therapeutics, Scholar Rock, Terns Pharmaceuticals, Altimmune, Omega Therapeutics, Kallyope, Rivus and others including several privately financed companies whose information is not regularly disclosed to the public.
We are not currently aware of any competitors to SBI-100 Ophthalmic Emulsion that are developing a CB1 agonist for the treatment of glaucoma and OH. Merck, Novartis, Abbvie, Bausch + Lomb, and Alcon are generally known competitors currently offering treatments for glaucoma and ocular hypertension.
Manufacturing
We do not own or operate manufacturing facilities and we rely on third-party contract manufacturing organizations to supply Skye with drugs for pre-clinical and clinical studies.
Nimacimab
Nimacimab is a monoclonal antibody and we have developed a manufacturing process under current good manufacturing practice ("cGMP") to produce batches of drug substance and drug product for pre-clinical and clinical studies. Drug substance for nimacimab will be produced by a contract manufacturer through recombinant DNA technology utilizing genetically engineered host cells, upstream cell culture processes and downstream purification methods as required to manufacture the drug substance. Drug product will be produced by a contract manufacturer whereby nimacimab drug substance will be formulated and filled in to pre-filled syringes.
11
SBI-100 Ophthalmic Emulsion
Manufacturing of SBI-100 OE's active pharmaceutical ingredient, SBI-100, has been conducted in the United States by contract manufacturers under cGMP. Formulation and packaging of SBI-100 OE for nonclinical and clinical use is manufactured by contract manufacturers with necessary controlled substance licenses with appropriate local, state and federal government agencies to conduct research, manufacture and distribute controlled substances like THC.
Intellectual Property
The success of most of our product candidates will depend in large part on our ability to:
•Obtain and maintain patent and other legal protection for the proprietary technology, inventions and improvements we consider important to our business
•Prosecute our patent applications and defend any issued patents we obtain
•Preserve the confidentiality of our trade secrets
•Operate without infringing the patents and proprietary rights of third parties.
We intend to continue to seek patent protection for certain of our product candidates, drug delivery systems, molecular modifications, as well as other proprietary technologies and their uses by filing patent applications in the United States and other selected global territories. We intend for these patent applications to cover, where possible, claims for composition of matter, medical uses, processes for isolation and preparation, processes for delivery and formulations.
We also rely upon trade secrets and know-how and continuing technological innovation to develop and maintain our proprietary and intellectual property position. We seek to protect our proprietary information in part using confidentiality agreements with our collaborators, scientific advisors, employees and consultants, and invention assignment agreements with our employees and selected consultants, scientific advisors and collaborators. The confidentiality agreements are designed to protect our proprietary information and, in the case of agreements or clauses requiring invention assignment, to grant us ownership of technologies that are developed through a relationship with a third party.
Nimacimab
The Company owns three granted U.S. patents, 32 granted foreign patents, and 25 pending US and foreign patent applications directed to the compositions of matter for the nimacimab antibody and molecular variants, and to methods of treatment and uses of nimacimab and its variants for treating a number of diseases responsive to the modulation of the CB1. The patents and applications, once granted, will expire between 2035 and 2036 and may be eligible for up to five years of extension (Hatch-Waxman).
SBI-100 and SBI-100 OE
As of the date of this Annual Report, we have licensed an invention from UM which include U.S. patents as well as a number of foreign counterparts, including the European Union, Japan, Canada and Australia. The patents that we license cover composition of matter and preparation of SBI-100, and their methods of use. The US patent for SBI-100 is expected to expire in 2029. Under our license agreement, UM retains ownership over the licensed patents and control over the maintenance and prosecution of the licensed patents and patent applications.
Licensing and Other Agreements
Nimacimab
Skye wholly owns nimacimab. There are no in- or out-licensing arrangements, no contingent value rights and no royalties payable or due to Skye relating to this molecule.
Under the terms of the securities purchase agreement (the "January PIPE SPA") entered into in connection with the January PIPE Financing, so long as the investors in the January PIPE Financing continue to beneficially own in the aggregate at least 40% of the securities issued in the January PIPE Financing (such securities, the "Closing Securities"), the Company may not transfer, license (other than in the ordinary course of business), encumber, or sell a royalty interest in any intellectual property relating to nimacimab unless Skye obtains the written consent of Qualified Investors that, together with their respective affiliates, beneficially own at least a majority of the then outstanding Closing Securities owned by the Qualified Investors and their respective affiliates. The term "Qualified Investors" means any investor that, together with its affiliates, continues to own at least 80% of the securities originally purchased by it under the January PIPE SPA.
12
SBI-100
University of Mississippi granted Skye an exclusive license for all fields of use related to UM 5050 (referred to by Skye as SBI-100) and including, with the prior written consent of UM, the right to sublicense the intellectual property for UM 5050. All fields of use means no restrictions on use of the underlying inventions, including developing UM 5050 to treat any disease through any form of delivery under the license agreement.
The license for SBI-100, a CB1 agonist, is expected to allow us to explore related uses for the active moiety of SBI-100.
In 2022, after notifying the Australian Therapeutics Goods Administration through the Clinical Trial Notification scheme of our intent to initiate our Phase 1 clinical trial for SBI-100 OE, we triggered and paid the first milestone payment under our License Agreement for UM 5050 with UM.
In 2023, we granted to Tautomer Bioscience an exclusive license to develop and commercialize SBI-100 as a novel suppository formulation for chronic intractable pain and other indications in South Africa and the rest of Africa. We have retained certain rights and options to obtain rights to the future use of new jointly developed intellectual property and other intellectual property owned or controlled by Tautomer Bioscience related to SBI-100.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in other countries, extensively regulate, among other things, the research, development, testing, manufacture, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, import and export of pharmaceutical products such as those we are developing. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources. A failure to comply with such laws and regulations or prevail in any enforcement action or litigation related to noncompliance could have a material adverse impact on our business, financial condition and results of operations and could cause the market value of our common stock to decline.
U.S. Food and Drug Administration (FDA)
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The FDA regulates drugs under the Federal Food, Drug and Cosmetic Act ("FDCA") and its implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject us to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending NDAs, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The process required by the FDA before a drug may be legally marketed in the United States, generally involves the following:
•conduct of laboratory tests, animal studies and formulation studies in compliance with GLP regulations;
•submission of an Investigational New Drug application ("IND") to the FDA , which must be found acceptable to proceed before clinical trials may begin;
•approval and oversight of each study by an IRB before each clinical site may initiate the trial(s);
•conduct of adequate and well-controlled clinical trials in accordance with good clinical practice ("GCP") requirements to establish the safety and efficacy of the proposed drug for each indication;
•submission of an NDA to the FDA;
•satisfactory development and completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP requirements and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity;
•satisfactory development and completion of an FDA BioResearch Monitoring (BIMO) inspection of the clinical study sites which participated in the studies supporting the NDA application; and
•FDA review and approval of marketing authorization application (NDA, BLA, etc).
13
Nonclinical Studies
Nonclinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess potential safety and efficacy. An IND sponsor must submit the results of the nonclinical tests, together with manufacturing information, analytical data and any available clinical data or literature, among other things, to the FDA as part of an IND. Some nonclinical testing may continue even after the IND is submitted. An IND generally becomes effective 30 days after receipt by the FDA unless, before that time, the FDA raises concerns or questions related to nonclinical or manufacturing documentation or one or more proposed clinical trials and places the IND on clinical hold. In such a case, the IND sponsor and the FDA work to resolve any outstanding concerns before the hold can be lifted and the clinical trial(s) can begin. As a result, submission of an IND does not always result in the FDA allowing clinical trials to commence.
Clinical Trials
Clinical trials involve the administration of the investigational new drug candidate to humans under the supervision of qualified investigators in accordance with GCP requirements. This includes the requirement that all research subjects/patients provide their informed consent in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB, either central or at each institution participating in the clinical trial must review and approve the plan for any clinical trial before the study commences at a site. Information about certain clinical trials must be submitted within specific timeframes to the NIH for public dissemination on the www.clinicaltrials.gov website.
Before marketing authorization, human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
•Phase 1: The drug is initially introduced into a small number of healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness.
•Phase 2: The drug is administered to a specific patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy and safety of the product for specific diseases and to determine dosage tolerance and optimal dosage.
•Phase 3: The drug is administered to the established patient population expected to benefit based upon the risk/benefit profile. Generally, this phase of studies are conducted at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
Reports detailing the results of the clinical trials must be submitted at least annually to the FDA and IND safety reports are submitted more frequently if serious adverse events possibly related to the investigational product occur. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the Sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk or due to a business decision. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Marketing Approval
Assuming successful completion of the required clinical testing, the results of the nonclinical and clinical studies, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an marketing authorization application requesting approval to market the product for one or more indications. For drug products, or biologic products under the review of Center for Drug Evaluation and Research (CDER), the marketing authorization application is an NDA or a BLA, and submission is subject to a substantial application user fee. Under the Prescription Drug User Fee Act ("PDUFA") commitments that are currently in effect, the FDA has a goal of reviewing and responding to a submission within ten months from the date of “filing” of a standard NDA for a new molecular entity. This review typically takes at least twelve months from the date the NDA is submitted to the FDA because the FDA has approximately two months to make a “filing” decision. However, if issues arise during the review, the FDA may request additional information and the review period may be extended to permit the applicant to provide and the FDA to review that information, which may significantly extend this time period. The process for review and issuance of a license for a BLA is similar to the NDA review path..
14
In addition, under the Pediatric Research Equity Act of 2003 ("PREA"), as amended and reauthorized, certain marketing authorizations or supplements to marketing authorizations must contain data that is adequate to assess the safety and effectiveness of the approved product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements.
The FDA also may require submission of a Risk Evaluation and Mitigation Strategy (REMS) plan to ensure that the benefits of the drug outweigh its risks. The REMS plan could include medication guides, physician communication plans, assessment plans, and/or elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
The FDA conducts a preliminary review of all pending marketing authorizations within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may refuse to file the application and request additional information rather than accept marketing authorization application for filing. In this event, the application must be resubmitted with the additional information requested. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA reviews an NDA to determine, among other things, whether the drug is safe and effective and whether the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, quality and purity.
The FDA may refer an application for a novel product and/or first-in-class product to an advisory committee. The FDA may also refer to the advisory committee certain scientific questions raised by an application. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving a marketing authorization application, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, within the review period and before approving a marketing authorization application, the FDA will likely inspect one or more clinical trial sites to assure compliance with GCP requirements.
The testing and approval process for an NDA/BLA requires substantial time, effort and financial resources, and each may take several years to complete. Data obtained from nonclinical and clinical testing are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all.
After evaluating the NDA/BLA and all related information, including the advisory committee recommendation, if any, and inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter, or, in some cases, a complete response letter. A complete response letter generally contains a statement of specific conditions that must be met to secure final approval of the NDA/BLA and may require additional clinical or nonclinical testing in order for the FDA to reconsider the application. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter for the NDA/BLA. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. For some products, such as our product candidates where abuse potential is a possibility, an additional step of Drug Enforcement Administration (DEA) review and scheduling is required.
Post-Approval Requirements
Products manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion, and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to FDA review and approval prior to implementation. There also are continuing annual product fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA/BLA. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
15
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP requirements and impose reporting and documentation requirements upon the sponsor and any third party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market.
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in mandatory revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program.
Other potential consequences include, among other things:
•restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
•fines, warning letters or holds on post-approval clinical trials;
•refusal of the FDA to approve pending NDAs/BLAs or supplements to approved NDAs/BLAs, or suspension or revocation of product licenses or approvals;
•product seizure or detention, or refusal to permit the import or export of products; or
•injunctions or the imposition of civil or criminal penalties.
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act ("PDMA"), which regulates the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Exclusivity and Approval of Competing Products
Hatch Waxman Act
Section 505 of the FDCA describes three types of marketing applications that may be submitted to the FDA to request marketing authorization for a new drug. A Section 505(b)(1) NDA is an application that contains full reports of investigations of safety and efficacy. A 505(b)(2) NDA is an application that contains full reports of investigations of safety and efficacy but where at least some of the information required for approval comes from investigations that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted. This regulatory pathway enables the applicant to rely, in part, on the FDA’s prior findings of safety and efficacy for an existing product, or published literature, in support of its application. Section 505(j) establishes an abbreviated approval process for a generic version of approved drug products through the submission of an Abbreviated New Drug Application ("ANDA"). An ANDA provides for marketing of a generic drug product that has the same active ingredients, dosage form, strength, route of administration, labeling, performance characteristics and intended use, among other things, to a previously approved product. ANDAs are termed “abbreviated” because they are generally not required to include preclinical (animal) and clinical (human) data to establish safety and efficacy. Instead, generic applicants must scientifically demonstrate that their product is bioequivalent to, or performs in the same manner as, the innovator drug through in vitro, in vivo, or other testing. The generic version must deliver the same amount of active ingredients into a subject’s bloodstream in the same amount of time as the innovator drug and can often be substituted by pharmacists under prescriptions written for the reference listed drug.
16
Hatch Waxman Patent Exclusivity
In seeking approval for a drug through a NDA, applicants are required to list with the FDA each patent with claims that cover the applicant’s product or a method of using the product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential competitors in support of approval of an ANDA or 505(b)(2) NDA.
The ANDA or 505(b)(2) NDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval. Specifically, the applicant must certify with respect to each patent that:
•the required patent information has not been filed;
•the listed patent has expired;
•the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or
•the listed patent is invalid, unenforceable or will not be infringed by the new product.
Generally, the ANDA or 505(b)(2) NDA cannot be approved until all listed patents have expired, except when the ANDA or 505(b)(2) NDA applicant challenges a listed drug. A certification that the proposed product will not infringe the already approved product’s listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicate that it is not seeking approval of a patented method of use, the ANDA or 505(b)(2) NDA application will not be approved until all the listed patents claiming the referenced product have expired.
If the ANDA or 505(b)(2) NDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the application has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of notice of the Paragraph IV certification automatically prevents the FDA from approving the ANDA or 505(b)(2) NDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant.
Hatch Waxman Non-Patent Exclusivity
In addition to patent issues, market and data exclusivity provisions under the FDCA can delay the submission or the approval of certain applications for competing products. The FDCA provides a five-year period of non-patent data exclusivity within the United States to the first applicant to gain approval of a NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the activity of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submitted by another company that references the previously approved drug. However, an ANDA or 505(b)(2) NDA may be submitted after four years if it contains a Paragraph IV certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for a NDA, 505(b)(2) NDA, or supplement to an existing NDA or 505(b)(2) NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant, are deemed by the FDA to be essential to the approval of the application or supplement. Three-year exclusivity may be awarded for changes to a previously approved drug product, such as new indications, dosages, strengths or dosage forms of an existing drug.
This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and, as a general matter, does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for other versions of a drug. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a disease or condition that affects populations of fewer than 200,000 individuals in the United States or, if it affects more than 200,000 individuals in the United States, there is no reasonable expectation that the cost of developing and making a drug product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan designation must be requested before submitting a NDA. Orphan designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
17
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity or inability to manufacture the product in sufficient quantities. The designation of such drug also entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. Competitors, however, may receive approval of different products for the same indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication than that for which the orphan product has exclusivity.
Regulation of Controlled Substances: Drug Enforcement Administration (DEA) Regulation
Certain cannabinoids are regulated as “controlled substances” as defined in the Controlled Substances Act (the “CSA”), which establishes registration, security, recordkeeping, reporting, storage, distribution and other requirements administered by the DEA. The DEA is concerned with the control of handlers of controlled substances (and with the equipment and raw materials used in their manufacture and packaging) of controlled substances in order to prevent loss and diversion into illicit channels of commerce.
The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use and may not be marketed or sold in the United States. A pharmaceutical product may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances. Certain cannabinoids are listed by the DEA as Schedule I controlled substances under the CSA. Consequently, their manufacture, shipment, storage, sale and use are subject to a high degree of regulation. Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which schedules of controlled substances are authorized.
The DEA typically inspects a facility to review its security measures prior to issuing a registration. Security requirements vary by controlled substance schedule, with the most stringent requirements applying to Schedule I and Schedule II substances. Required security measures include background checks on employees and physical control of inventory through measures such as cages, surveillance cameras and inventory reconciliations. The registered entity must maintain records for the handling of all controlled substances and must make periodic reports to the DEA. These include, for example, distribution reports for Schedule I and II controlled substances, Schedule III substances that are narcotics, and other designated substances. The registered entity must also report thefts or losses of any controlled substance and obtain authorization to destroy any controlled substance. In addition, special authorization and notification requirements apply to imports and exports.
In addition, a DEA quota system controls and limits the availability and production of controlled substances in Schedule I or II. Distributions of any Schedule I or II controlled substance must also be accompanied by special order forms, with copies provided to the DEA. The DEA may adjust aggregate production quotas and individual production and procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments. To meet its responsibilities, the DEA conducts periodic inspections of registered establishments that handle controlled substances. In the event of non-compliance, the DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
SBI-100 remains a Schedule I controlled substance, pending a request to re-schedule SBI-100 after marketing authorization by the FDA or execution of the August 23, 2023 request by the Assistant HHS secretary to DEA reschedule cannabis to Schedule 3.
Federal and State Fraud and Abuse, Data Privacy and Security Laws and Regulations
In addition to FDA restrictions on marketing of pharmaceutical products, federal and state fraud and abuse laws restrict business practices in the pharmaceutical industry. These laws include anti-kickback and false claims laws and regulations as well as data privacy and security laws and regulations.
18
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering, or arranging for or recommending the purchase, lease, or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exemptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not meet the requirements of a statutory or regulatory exception or safe harbor. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
The federal False Claims Act prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. A violation of the federal Anti-Kickback Statute also constitutes a false or fraudulent claim for purposes of the civil False Claims Act.
Several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of products for unapproved, and thus non-covered, uses. In addition, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
The federal False Claims Act also created federal criminal statutes that prohibit knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Pharmaceutical companies are also subject to the civil monetary penalties statute, which imposes penalties against any person who is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and other health care providers. The Patient Protection and Affordable Care Act, as amended by the ACA, signed into law on March 2010, created new federal requirements for reporting, by applicable manufacturers of covered drugs, payments and other transfers of value to physicians and teaching hospitals. Applicable manufacturers are also required to report annually to the government certain ownership and investment interests held by physicians and their immediate family members. In addition, certain states require implementation of commercial compliance programs and compliance with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, impose restrictions on marketing practices, and/or tracking and reporting of gifts, compensation and other remuneration or items of value provided to physicians and other health care professionals and entities.
We may also be subject to data privacy and security obligations, including federal and state laws related to the privacy and security of personal information. The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and its implementing regulations (the “HIPAA Rules”) imposes specified requirements relating to the privacy, security and transmission of protected health information (“PHI”) and may apply to certain of the information we process. Among other things, HITECH makes HIPAA’s security and certain of its privacy requirements directly applicable to “business associates,” defined as independent contractors or agents of covered entities, or other business associates, that create, receive, maintain, obtain, or transmit PHI in connection with providing a service on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same requirements, thus complicating compliance efforts.
19
To the extent that any of our product candidates, once approved, are sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or other transfers of value to healthcare professionals.
The shifting commercial compliance environment and the need to build and maintain robust systems to comply with different compliance and/or reporting requirements in multiple jurisdictions increase the possibility that a healthcare company may violate one or more of the requirements. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we obtain regulatory approval. In the United States and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend, in part, on the availability of coverage and reimbursement from third party payors. Third party payors include government authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the reimbursement rate that the payor will pay for the drug product. Third party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular indication. A decision by a third party payor not to cover our products, if approved, could reduce physician utilization of our products once approved and have a material adverse effect on our sales, results of operations and financial condition. Moreover, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. By way of example, in the United States, the ACA contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries, and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, reform government program reimbursement methodologies. For example, on August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. For that and other reasons, it is currently unclear how the IRA will be effectuated. Additional state and federal healthcare reform measures may be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products once approved or additional pricing pressures.
Foreign Regulation
In order to market any product outside of the United States, we must comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials and commercial sales and distribution of our products. While our management and many of our consultants are familiar with and have been responsible for gaining marketing approval in many countries, we have not reviewed the specific regulations in countries outside of the United States, as it pertains to cannabinoids.
Additional Regulation
We are a reporting company with the Securities and Exchange Commission (the "SEC"), and, therefore, subject to the information and reporting requirements of the Exchange Act of 1934, as amended (the "Exchange Act") and other federal securities laws, and the compliance obligations of the Sarbanes-Oxley Act of 2002 ("Sarbanes-Oxley Act"). In addition, our financial reporting is subject to United States Generally Accepted Accounting Principles ("GAAP"), and GAAP is subject to change over time.
20
We are also subject to federal, state and local laws and regulations applied to businesses generally. We believe that we are in conformity with all applicable laws in all relevant jurisdictions.
Employees & Human Capital Resources
As of the date of this Annual Report, we have a total of eleven full-time employees. None of our employees are represented by a labor union or covered by a collective bargaining agreement. The majority of our employees work remotely, which we believe has provided us with greater access to qualified personnel around the United States. At the Company, we strive to foster collaborative, communicative and flexible environment so that our employees feel supported in the workplace.
We anticipate that we will need to hire additional employees or independent contractors for our continued development efforts. We also intend to utilize independent contractors and outsourced services, such as CROs and third party manufacturers, where possible and appropriate.
Corporate Information
We were incorporated in the State of Nevada on March 16, 2011.
Our principal executive office is located at 11250 El Camino Real, Suite 100, San Diego, CA 92130. Our telephone number is (858) 410-0266.
Our Internet website, which is located at http://www.skyebioscience.com, describes our company and our management and provides information about our technology and products. Information contained on our website is not incorporated by reference into, and should not be considered a part of, this Annual Report.
Available Information
Our filings, including Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments submitted under Sections 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, are accessible at no cost on our company website promptly after submission to the Securities and Exchange Commission ("SEC"). Additionally, these documents are retrievable from the SEC's website (www.sec.gov).
Corporate governance materials, such as our guidelines and committee charters, are also accessible on our investor relations webpage under "Corporate Governance." It's important to note that the content of our websites is not intended for inclusion by reference in our filings with the SEC, and any website references serve as inactive textual mentions only.
RISK FACTORS
Item 1A. Risk Factors.
Investing in our common stock, involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information contained in this Annual Report, including our consolidated financial statements and their related notes included elsewhere in this Annual Report and Part II. Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” before making an investment decision. If any of the following risks actually occurs, our business, prospects, operating results and financial condition could suffer materially, the trading price of our common stock could decline and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial also may materially and adversely affect our business, prospects, operating results and financial condition.
Risk Factor Summary
Below is a summary of the principal factors that make an investment in our common stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below under the heading “Risk Factors” and should be carefully considered, together with other information in this Annual Report on Form 10-K and our other filings with the Securities and Exchange Commission, or the SEC, before making an investment decision regarding our common stock.
21
Risks Related to Our Limited Operating History, Financial Position and Capital Requirements
We have a limited operating history, have incurred significant operating losses since our inception and expect to incur significant losses for the foreseeable future. We may never generate any revenue or become profitable or, if we achieve profitability, we may not be able to sustain it.
Pharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We are a clinical-stage pharmaceutical company with a limited operating history upon which you can evaluate our business and prospects. We commenced operations in 2011, and to date, we have focused primarily on organizing and staffing our company, business planning, raising capital, discovering potential product candidates, and conducting preclinical studies and clinical trials. Our approach to the discovery and development of product candidates is unproven, and we do not know whether we will be able to develop any products of commercial value. In addition, we have only two product candidates, SBI-100 and nimacimab, in clinical development. We have not yet demonstrated an ability to obtain marketing approval for any of our product candidates, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, any predictions made about our future success or viability may not be as accurate as they could be if we had a history of successfully developing and commercializing pharmaceutical products.
We have incurred significant operating losses since our inception. If our product candidates are not successfully developed and approved, we may never generate any revenue. We have incurred cumulative net losses since our inception and, as of December 31, 2023, we had an accumulated deficit of $104,382,549 . Our losses have primarily resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations. All of our product candidates will require substantial additional development time and resources before we would be able to apply for or receive regulatory approvals and begin generating revenue from product sales. We expect to continue to incur losses for the foreseeable future, and we anticipate these losses will increase substantially as we continue our development of, seek regulatory approval for and potentially commercialize any approved products.
22
To become and remain profitable, we must succeed in developing and eventually commercializing or licensing products that generate significant revenue. This will require us to be successful in a range of challenging activities, including clinical trials of our product candidates, discovering additional product candidates, obtaining regulatory approval for these product candidates and manufacturing, marketing and selling any products for which we may obtain regulatory approval. We may never succeed in these activities and, even if we do, may never generate revenues that are significant enough to achieve profitability. In addition, we have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical industry. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our product candidates or even continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our product development programs, commercialization efforts or other operations.
The development of biopharmaceutical product candidates and conducting preclinical studies and clinical trials are time-consuming and capital-intensive. We expect our expenses to increase in connection with our ongoing activities, particularly as we conduct our ongoing and planned Phase 2 clinical trials of SBI-100 and nimacimab and continue our research and development activities. Furthermore, we incur, and expect to continue to incur, additional costs associated with operating as a public company. At the same time, our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
We believe that our existing cash, cash equivalents and investment securities will enable us to fund our operations for at least the next 12 months. We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect. Our operating plans and other demands on our cash resources may change as a result of many factors currently unknown to us. Because the outcome of any preclinical study or clinical trial is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our product candidates. Our future capital requirements will depend on many factors, including:
•the type, number, scope, progress, expansions, results, costs and timing of our preclinical studies and clinical trials of our product candidates which we are pursuing or may choose to pursue in the future;
•the costs and timing of manufacturing and laboratory testing for our product candidates, including clinical supplies and commercial manufacturing if any product candidate is approved;
•the costs, timing and outcome of regulatory review of our product candidates;
•the costs of obtaining, maintaining and enforcing our patents and other intellectual property rights;
•our efforts to enhance operational systems and hire additional personnel to satisfy our obligations as a public company, including enhanced internal controls over financial reporting;
•the costs associated with hiring additional and retaining existing personnel and consultants as our preclinical and clinical activities increase;
•the costs and timing of establishing or securing sales and marketing capabilities if any product candidate is approved;
•our ability to achieve sufficient market acceptance, adequate coverage and reimbursement from third-party payors and adequate market share and revenue for any approved products;
•the effect of competing technological and market developments;
•the terms and timing of establishing and maintaining collaborations, licenses and other similar arrangements;
•costs associated with any products or technologies that we may in-license or acquire; and
•the funding of any co-development arrangements we enter into.
23
Accordingly, we may need to seek additional funds sooner than planned, including through public or private equity or debt financings or other sources or through strategic collaborations. Attempting to secure additional financing may divert our management from our day-to-day activities, which may adversely affect our ability to develop our product candidates. Adequate additional financing may not be available to us on acceptable terms, or at all. We do not currently have any active grants nor do we expect grant revenues to be a material source of future revenue. If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs, including our clinical trial programs, or any future commercialization of any product candidates, or be unable to sustain or expand our operations or otherwise capitalize on our business opportunities, as desired, any of which could materially affect our business, financial condition and results of operations.
Our ability to raise capital may be limited by applicable laws and regulations.
Using a shelf registration statement on Form S-3 to raise additional capital generally takes less time and is less expensive than other means, such as conducting an offering under a Form S-1 registration statement. However, our ability to raise capital using a shelf registration statement may be limited by, among other things, SEC rules and regulations. Under SEC rules and regulations, if our public float (the market value of our common stock held by non-affiliates) is less than $75,000,000, then the aggregate market value of securities sold by us or on our behalf under our Form S-3 in any 12-month period is limited to an aggregate of one-third of our public float. While our public float is currently more than $75,000,000, we have been subject to this limitation in the past and we may be subject to it again in the future. If our ability to utilize a Form S-3 registration statement for a primary offering of our securities is limited to one-third of our public float, we may conduct such an offering pursuant to an exemption from registration under the Securities Act or under a Form S-1 registration statement, and we would expect either of those alternatives to increase the cost of raising additional capital relative to utilizing a Form S-3 registration statement.
The sale of additional shares or other equity securities could result in additional dilution to our stockholders.
We require additional capital for the development and commercialization of our product candidates and may require additional cash resources due to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our resources are insufficient to satisfy our cash requirements, we will seek to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities could result in additional dilution to our stockholders. If we incur additional indebtedness it would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all.
Risks Related to the Discovery, Development and Regulatory Approval of Our Product Candidates
We are heavily dependent on the success of our early-stage product candidates, SBI-100 and nimacimab, which will require significant additional efforts to develop and may prove not to be viable for commercialization.
We have no products approved for sale and all of our product candidates are in clinical development. Our business depends entirely on the successful development, clinical testing, and commercialization of these and any other product candidates we may seek to develop in the future, which may never occur.
The success of our product candidates will depend on several factors, any one of which we may not be able to successfully complete, such as:
•With respect to SBI-100, receipt of necessary controlled substance registrations from the DEA;
•successful completion of preclinical studies and clinical trials;
•approval from regulatory agencies, such as the FDA or an IRB, to conduct our clinical trials;
•receipt of marketing approvals from the FDA and other applicable regulatory authorities;
•obtaining, maintaining and protecting our intellectual property portfolio, including patents and trade secrets, and regulatory exclusivity for our product candidates;
•identifying, making arrangements and ensuring necessary registrations with third-party manufacturers, or establishing commercial manufacturing capabilities for applicable product candidates;
•launching commercial sales of the products, if and when approved, whether alone or in collaboration with others;
•acceptance of our products, if and when approved, by patients, the medical community and third-party payors;
•effectively competing with other therapies;
24
•obtaining and maintaining healthcare coverage and adequate reimbursement of our products; and
•maintaining a continued acceptable safety profile of our products following approval.
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would materially harm our business.
Due to our limited resources, we may be forced to focus on a limited number of development candidates which may force us to pass on opportunities that could have a greater chance of clinical success.
Due to our limited resources and capabilities, we will have to decide to focus on developing a limited number of product candidates, currently SBI-100 and nimacimab. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial product candidates or profitable market opportunities. Our spending on research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
If we fail to demonstrate the safety and efficacy of any product candidate that we develop to the satisfaction of the regulatory authorities, we may incur additional costs or experience difficulty in completing, the development and commercialization of such product candidate.
We are not permitted to commercialize, market, promote, or sell any product candidate in the United States without obtaining marketing approval from the FDA or in other countries without obtaining approvals from comparable foreign regulatory authorities, such as the European Medicines Agency, and we may never receive such approvals. To gain approval to market a drug product, we must complete extensive nonclinical development and clinical trials that demonstrate the safety and efficacy of the product for the intended indication to the satisfaction of the FDA or other regulatory authority.
We have not previously submitted a NDA to the FDA, or similar drug approval filings to comparable foreign authorities, for any product candidate, and we cannot be certain that any of our product candidates will be successful in clinical trials or receive regulatory approval. If we do not receive regulatory approval for our product candidates, we may not be able to continue our operations. Even if we successfully obtain regulatory approval to market our product candidates, our revenue will be dependent, in part, upon the size of the markets in the territories for which we gain regulatory approval and have commercial rights.
The FDA or any foreign regulatory bodies could delay, limit or deny approval of our product candidates for many reasons, including our inability to demonstrate to the satisfaction of the FDA or the applicable foreign regulatory body that the product candidate is safe and effective for the requested indication, the regulatory agency’s disagreement with the interpretation of data from preclinical studies or clinical trials, or our inability to demonstrate that the clinical and other benefits of the product candidate outweigh any safety or other perceived risks. The FDA or applicable regulatory body could also require additional preclinical or clinical studies, deny approval of the formulation, labeling or the specifications of the product candidate, or the manufacturing processes or facilities of third party manufacturers with which we contract. The policies of the applicable regulatory agencies could also significantly change in a manner rendering our clinical data insufficient for approval.
Even if we eventually complete clinical testing and receive approval of a NDA or foreign regulatory filing for a product candidate, the FDA or the applicable foreign regulatory agency may grant approval contingent on the performance of costly additional clinical trials. The FDA or the applicable foreign regulatory agency also may approve the product candidate for a more limited indication or a narrower patient population than we originally requested, and the FDA, or applicable foreign regulatory agency, may not approve the labeling that we believe is necessary or desirable for the successful commercialization of the product. Any delay in obtaining, or inability to obtain, applicable regulatory approval would delay or prevent commercialization of the product candidate and would materially adversely impact our business and prospects.
25
Nonclinical and clinical drug development involves a lengthy and expensive process with an uncertain outcome. We may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
Clinical testing is expensive and can take several years to complete, and its outcome is inherently uncertain. Moreover, obtaining sufficient quantities of certain product of SBI-100 for clinical testing is subject to regulation by DEA and, in some cases, NIDA (National Institute of Drug Abuse). It is impossible to predict when or if any of our product candidates will prove effective or safe in humans or will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, we must complete preclinical studies and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. A failure of one or more clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive marketing approval or subsequently to commercialize our product candidates, including:
•FDA, DEA or NIDA or other foreign equivalent authorities may not authorize the use and distribution of sufficient quantities of product for clinical testing;
•regulators or independent institutional review boards (IRBs) may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
•we may experience delays in reaching, or fail to reach, agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites;
•regulators or IRBs may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;
•the cost of clinical trials of our product candidates may be greater than we anticipate;
•our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or IRBs to suspend or terminate the trials.
If we experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. In addition, some of our competitors have ongoing clinical trials for product candidates that treat the same indications as our product candidates, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates. Patient enrollment is affected by other factors including the severity of the disease under investigation, the eligibility criteria for the study in question, the perceived risks and benefits of the product candidate, the patient referral practices of physicians, the ability to monitor patients adequately during and after treatment, and the proximity and availability of clinical trial sites for prospective patients.
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays and could require us to abandon one or more clinical trials altogether, which could result in increased development costs and cause the value of our company to decline and limit our ability to obtain additional financing.
26
Our development and commercialization strategy for SBI-100 OE, may depend, in part, on published scientific literature and the FDA’s prior findings regarding the safety and efficacy of dronabinol, based on data not developed by us, but upon which the FDA may rely in reviewing our NDA.
The Hatch-Waxman Act added Section 505(b)(2) to the FDCA, Section 505(b)(2) permits the filing of a NDA where at least some of the information required for approval comes from investigations that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted. The FDA interprets Section 505(b)(2) of the FDCA, for purposes of approving a NDA, to permit the applicant to rely, in part, upon published literature or the FDA’s previous findings of safety and efficacy for an approved product. The FDA may also require companies to perform additional clinical trials or measurements to support any deviation from the previously approved product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant. The label, however, may require all or some of the limitations, contraindications, warnings or precautions included in the listed product’s label, including a black box warning, or may require additional limitations, contraindications, warnings or precautions. Depending on guidance from the FDA, we may decide to submit a NDA for SBI-100 under Section 505(b)(2) relying, in part, on the FDA’s previous findings of safety and efficacy from investigations for the approved drug product dronabinol for which we have not received a right of reference and published scientific literature. Even though we may be able to take advantage of Section 505(b)(2) to support potential U.S. approval, the FDA may require us to perform additional clinical trials or measurements to support approval. In addition, notwithstanding the approval of many products by the FDA pursuant to Section 505(b)(2), some pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA changes its interpretation of Section 505(b)(2), or if the FDA’s interpretation is successfully challenged in court, this could delay or even prevent the FDA from approving any Section 505(b)(2) NDAs that we submit. Such a result could require us to conduct additional testing and costly clinical trials, which could substantially delay or prevent the approval and launch of SBI-100 or future product candidates.
Serious adverse events or undesirable side effects or other unexpected properties of any of our product candidates may be identified during development or after approval that could delay, prevent or cause the withdrawal of marketing approval, limit the commercial potential, or result in significant negative consequences following marketing approval.
Serious adverse events or undesirable side effects caused by, or other unexpected properties of, our product candidates could cause us, an IRB, or regulatory authorities to interrupt, delay or halt our clinical trials and could result in a more restrictive label, the imposition of distribution or use restrictions or the delay or denial of regulatory approval by the FDA or comparable foreign regulatory authorities. If any of our product candidates are associated with serious adverse events or undesirable side effects or have properties that are unexpected, we may need to abandon their development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. In our completed Phase 1 study of nimacimab, reported treatment emergent adverse events were diarrhea, headache, dizziness, upper respiratory tract infection, nausea and vomiting. In our completed Phase 1 study of SBI-100, the following adverse events occurred and were considered probably or possibly related to the study drug, included discomfort and pain upon eye drop instillation and mild hyperaemia. However, further analysis may reveal adverse events inconsistent with the safety results observed. Many compounds that initially showed promise in clinical or earlier stage testing have later been found to cause undesirable or unexpected side effects that prevented further development of the compound.
Undesirable side effects or other unexpected adverse events or properties of any of our other product candidates could arise or become known either during clinical development or, if approved, after the approved product has been marketed. If such an event occurs during development, our trials could be suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development of, or deny approval of, our product candidates. If such an event occurs after such product candidates are approved, a number of potentially significant negative consequences may result, including withdrawal of regulatory approval, requirements for additional warnings on the label, use or distribution restrictions, requirements to conduct post-market studies, requirements to create a medication guide outlining side effects, and liability for harm caused to patients.
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidate, if approved, or could substantially increase commercialization costs and expenses, which could delay or prevent us from generating revenue from the sale of our products and harm our business and results of operations.
27
As an organization, we have never conducted later-stage clinical trials or submitted an NDA or BLA, and may be unable to do so for any of our product candidates.
We are early in our development efforts for our product candidates, and we will need to successfully complete pivotal clinical trials in order to seek FDA or applicable foreign authority approval to market SBI-100, nimacimab and any future product candidates we may develop. Carrying out clinical trials and the submission of NDAs and BLAs are complicated. Based on the stage of development of our product candidates, the Company has not conducted any later stage or pivotal clinical trials. We also plan to conduct a number of clinical trials for multiple product candidates in parallel over the next several years. This may be a difficult process to manage with our limited resources and may divert the attention of management. In addition, we cannot be certain how many clinical trials of our product candidates will be required or how such trials will have to be designed to obtain marketing authorization. Consequently, we may be unable to successfully and efficiently execute and complete necessary clinical trials in a way that leads to regulatory submission and approval of any of our product candidates. We may require more time and incur greater costs than our competitors and may not succeed in obtaining marketing approvals of product candidates that we develop. Failure to commence or complete, or delays in, our planned clinical trials, could prevent us from or delay us in submitting NDAs for and commercializing our product candidates.
We have conducted clinical trials for our product candidates outside of the United States and we may do so for our product candidates in the future. However, the FDA and other foreign equivalents may not accept data from such trials, in which case our development plans will be delayed, which could materially harm our business.
We have conducted our initial Phase 1 clinical trial for SBI-100 OE in Australia. The acceptance of study data from clinical trials conducted outside the U.S. or another jurisdiction by the FDA or a comparable foreign regulatory authority may be subject to certain conditions or may not be accepted at all. For example, in cases where data from foreign clinical trials are intended to serve as the sole basis for marketing approval in the U.S., the FDA will generally not approve the application on the basis of foreign data alone unless (i) the data are applicable to the U.S. population and U.S. medical practice; (ii) the trials were performed by clinical investigators of recognized competence and pursuant to GCP regulations; and (iii) the data may be considered valid without the need for an on-site inspection by the FDA, or if the FDA considers such inspection to be necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means. In addition, even where the foreign study data are not intended to serve as the sole basis for approval, the FDA will not accept the data as support for an application for marketing approval unless the study is adequately designed and well-controlled, conducted in accordance with GCP requirements and the FDA is able to validate the data from the study through an onsite inspection if deemed necessary. Many foreign regulatory authorities have similar approval requirements. In addition, such foreign trials would be subject to the applicable local laws of the foreign jurisdictions where the trials are conducted.
Conducting trials outside the United States also exposes us to additional risks, including risks associated with:
•additional foreign regulatory requirements;
•foreign exchange fluctuations;
•compliance with foreign manufacturing, customs, shipment and storage requirements;
•cultural differences in medical practice and clinical research;
•diminished protection of intellectual property in some countries; and
•interruptions or delays in our trials resulting from geopolitical events, such as war or terrorism.
Preliminary, topline and interim data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publicly disclose interim, preliminary or topline data from our clinical trials, which are based on a preliminary analysis of then-available data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the topline or preliminary results that we report may differ from future results of the same studies, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Topline and preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from the topline or preliminary data we previously made public. As a result, topline and preliminary data should be viewed with caution until the final data are available. From time to time, we may also disclose interim data from our clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Adverse differences between topline, preliminary or interim data and final data could significantly harm our business prospects.
28
Further, others, including regulatory agencies, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could impact the value of the particular program, the approvability or commercialization of the particular product candidate or product and our company in general. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product, product candidate or our business. If the topline or preliminary data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed, which could harm our business, operating results, prospects or financial condition.
Disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being developed, approved or commercialized in a timely manner or at all, which could negatively impact our business.
The ability of the FDA and applicable foreign authorities to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the FDA have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government shut down several times and certain regulatory agencies, such as the FDA, furloughed critical employees and ceased critical activities.
Separately, in response to the COVID-19 pandemic, the FDA postponed most inspections of domestic and foreign manufacturing facilities at various points. Even though the FDA has since resumed standard inspection operations of domestic facilities where feasible, any resurgence of the virus or emergence of new variants may lead to further inspectional delays. Further, regulatory authorities outside the United States may adopt similar restrictions or other policy measures in response to the COVID-19 pandemic or any other pandemic or outbreak of a contagious disease. If a prolonged government shutdown occurs, or if global health concerns prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
Third parties may obtain FDA regulatory exclusivity to our detriment.
We plan to seek to obtain market exclusivity for our drug candidates and any other drug candidates we develop in the future. To the extent that patent protection is not available or has expired, FDA marketing exclusivity may be the only available form of exclusivity available for these proposed products. Marketing exclusivity can delay the submission or the approval of certain marketing applications. Potentially competitive products may also seek marketing exclusivity and may be in various stages of development, including some more advanced than our drug candidates. We cannot predict with certainty the timing of FDA approval or whether FDA approval will be granted, nor can we predict with certainty the timing of FDA approval for competing products or whether such approval will be granted. It is possible that competing products may obtain FDA approval with marketing exclusivity before we do, which could delay our ability to submit a marketing application or obtain necessary regulatory approvals, result in lost market opportunities with respect to our drug candidates and materially adversely affect our business, financial condition and results of operations.
29
Risks Related to Our Reliance on Third Parties
We expect to rely on third parties, such as CROs, to conduct some or all of our nonclinical and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize any of our product candidates.
We expect to rely on medical institutions, clinical investigators, contract laboratories and other third parties, such as CROs, to conduct our nonclinical and clinical studies on our product candidates in compliance with applicable regulatory requirements. For example, we are currently engaged with a CRO in the United States, to conduct our Phase 2 clinical study for SBI-100 OE . These third parties will not be our employees and, except for restrictions imposed by our contracts with such third parties, we will have limited ability to control the amount or timing of resources that they devote to our programs. Although we expect to rely on these third parties to conduct our preclinical studies and clinical trials, we will remain responsible for ensuring that each of our preclinical studies and clinical trials is conducted in accordance with its investigational plan and protocol and the applicable legal, regulatory, and scientific standards, and our reliance on these third parties will not relieve us of our regulatory responsibilities. In the case of our Phase 2 trial for SBI-100 OE, these entities must maintain and comply with valid DEA registrations and requirements. The FDA and regulatory authorities in other jurisdictions require us to comply with regulations and standards, commonly referred to as current good clinical practices, for conducting, monitoring, recording and reporting the results of clinical trials, in order to ensure that the data and results are scientifically credible and accurate and that the trial subjects are adequately informed of the potential risks of participating in clinical trials. If we or any of our third party contractors fail to comply with applicable current good clinical practices, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. In addition, we are required to report certain financial interests of our third party investigators if these relationships exceed certain financial thresholds and meet other criteria. The FDA or comparable foreign regulatory authorities may question the integrity of the data from those clinical trials conducted by principal investigators who previously served or currently serve as scientific advisors or consultants to us from time to time and receive cash compensation in connection with such services. Our clinical trials must also generally be conducted with products produced under current good manufacturing practice regulations. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
Some of the third parties with whom we contract may also have relationships with other commercial entities, some of which may compete with us. If the third parties conducting our preclinical studies or our clinical trials do not perform their contractual duties or obligations or comply with regulatory requirements, we may need to enter into new arrangements with alternative third parties. This could be costly, and our nonclinical studies or clinical trials may need to be extended, delayed, terminated or repeated, and we may not be able to obtain regulatory approval in a timely fashion, or at all, for the applicable product candidate, or to commercialize such product candidate being tested in such studies or trials. If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative third party contractors or to do so on commercially reasonable terms. Though we plan to carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
We rely on, and expect to continue relying on, third party contract manufacturing organizations to manufacture and supply product candidates for us, as well as certain raw materials used in the production thereof. If one of our suppliers or manufacturers fails to perform adequately, we may be required to incur significant delays and costs to find new suppliers or manufacturers.
We do not own facilities for, manufacturing our product candidates. We rely on, and expect to continue relying upon, third party manufacturing organizations to manufacture and supply our product candidates and certain raw materials used in the production thereof. Some of our key components for the production of our product candidates may have a limited number of suppliers.
30
The facilities used by our contract manufacturers to manufacture our product candidates must be approved by the FDA pursuant to inspections that will be conducted after we submit our NDA to the FDA. We expect that we will not control the manufacturing process of, and will be completely dependent on, our contract manufacturing partners for compliance with current good manufacturing practice requirements, for manufacture of our drug products. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of, as applicable, the FDA, DEA or others, they will not be able to secure and/or maintain DEA registrations and regulatory approval for their manufacturing facilities. In addition, we expect that we will have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our product candidates, or, with respect to SBI-100, if DEA does not register these facilities for the manufacture of controlled substances, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved.
Although we have quality agreements governing our development of clinical supplies, we do not have any commercial supply agreements with our suppliers. In the event that we and our suppliers cannot agree to the terms and conditions for them to provide clinical and commercial supply needs, we would not be able to manufacture our product or candidates until a qualified alternative supplier is identified, which could also delay the development of, and impair our ability to commercialize, our product candidates. The failure of third party manufacturers or suppliers to perform adequately or the termination of our arrangements with any of them may adversely affect our business.
If we fail to enter and maintain successful collaborative arrangements or strategic alliances for our product candidates, we may have to reduce or delay our product candidate development or increase our expenditures.
An important element of our strategy for developing, manufacturing and commercializing our product candidates is entering into collaborative arrangements or strategic alliances with pharmaceutical companies, research institutions or other industry participants to advance our programs and enable us to maintain our financial and operational capacity. As of the date of this filing, we have only one collaboration agreement with Tautomer Biosciences (Pty) Limited (“Tautomer”), pursuant to which, among other things, we granted to Tautomer an exclusive license to develop, manufacture and commercialize one or more products containing our proprietary amino acid ester prodrug of delta delta-9-tetrahydrocannabinol, in the licensed field in the countries of the continent of Africa and their territories and possessions. We face significant competition in seeking appropriate alliances. We may not be able to negotiate alliances on acceptable terms, if at all. In addition, these alliances may be unsuccessful. If we fail to create and maintain suitable alliances, we may have to limit the size or scope of, or delay, one or more of our research or development programs.
In addition, these kinds of collaborative arrangements and strategic alliances may place certain aspects of the development of our product candidates outside of our control, may require us to relinquish important rights or may otherwise be on terms unfavorable to us.
Dependence on collaborative arrangements or strategic alliances will subject us to several risks, including the risks that:
•we may not be able to control the amount and timing of resources that our collaborators may devote to the product candidates;
•a significant change in the senior management team, a change in the financial condition or a change in the business operations, including a change in control or internal corporate restructuring, of any of our collaborators, could result in delayed timelines, re-prioritization of our programs, decreasing resources or funding allocated to support our programs, or termination of the collaborations;
•we may be required to relinquish important rights such as marketing and distribution rights;
•business combinations or significant changes in a collaborator’s business strategy may also adversely affect a collaborator’s willingness or ability to complete its obligations under any arrangement;
•a collaborator could independently move forward with a competing product candidate developed either independently or in collaboration with others, including our competitors;
•collaborative arrangements are often terminated or allowed to expire, which would delay development and may increase the cost of developing our product candidates;
•collaborators may not comply with all applicable regulatory and legal requirements
31
Risks Related to Commercialization of Our Product Candidates
Even if we receive marketing approval for a product candidate, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and subject us to restrictions, withdrawal from the market, or penalties if we fail to comply with applicable regulatory requirements or if we experience unanticipated problems with our product candidates, when and if approved.
Once regulatory approval has been granted, the approved product and its manufacturer are subject to continual review by the FDA, the DEA (with respect to SBI-100 OE) and/or non-U.S. regulatory authorities and such approval may be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially costly post-marketing follow-up studies or surveillance. In addition, we will be subject to extensive and ongoing regulatory requirements with regard to labeling, packaging, adverse event reporting, storage, distribution, advertising, promotion, recordkeeping and submission of safety and other post-market information. Manufacturers of our products and manufacturers’ facilities are required to comply with current good manufacturing practice regulations, which include requirements related to quality control and quality assurance as well as the corresponding maintenance of records and documentation Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control. We will also be required to report certain adverse reactions and production problems, if any, to the FDA and to comply with requirements concerning advertising and promotion for our products. If we, any future collaboration partner or a regulatory authority discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory authority may impose restrictions on that product, the collaboration partner, the manufacturer or us, including requiring withdrawal of the product from the market or suspension of manufacturing.
Any DEA registrations that we receive for SBI-100 may also be subject to limitations such as the DEA’s annual manufacturing and procurement quota requirements. The annual quota allocated to us or our contract manufacturers for the controlled substances in our product candidates may not be sufficient to meet commercial demand. Our facilities that handle controlled substances, and those of our third party contractors, will also be subject to registration requirements and periodic inspections. Additionally, if approved by the FDA, the finished dosage forms of certain of SBI-100 OE will be subject to the DEA’s rescheduling process, which may delay product launch and impose additional regulatory burdens. Failure to maintain compliance with the CSA, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, financial condition and results of operations. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could lead to criminal proceedings. For additional information, see Risk Factor, “Our product candidate, SBI-100 OE, will be subject to U.S. controlled substance laws and regulations, and failure to comply with or the cost of compliance with these laws and regulations, may adversely affect the results of our business operations, and our financial condition.”
The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling and regulatory requirements. If we, our product candidates or the manufacturing facilities for our product candidates fail to comply with regulatory requirements of the FDA and/or other non-U.S. regulatory authorities, we could be subject to administrative or judicially imposed sanctions.
Widely publicized events concerning the safety risk of certain drug products have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products and the imposition by the FDA of risk evaluation and mitigation strategies, to ensure that the benefits of the drug outweigh its risks. In addition, widely publicized events concerning the safety risk of certain drug products have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products and the imposition by the FDA of risk evaluation and mitigation strategies to ensure that the benefits of the drug outweigh its risks. In addition, because of the serious public health risks of high-profile adverse safety events with certain products, the FDA may require, as a condition of approval, costly risk evaluation and mitigation strategies programs.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or in other countries. If we or any future collaboration partner are not able to maintain regulatory compliance, we or such collaboration partner, as applicable, will not be permitted to market our future products and our business will suffer.
32
We expect to face intense competition, often from companies with greater resources and experience than we have.
The highly competitive pharmaceutical industry continues to rapidly expand and evolve as an increasing number of competitors and potential competitors enter the market, many of which have substantially greater financial, technological, managerial and research and development resources and experience than we have. Our competitors have developed, are developing or may develop products, product candidates and processes competitive with our product candidates. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. We believe that a significant number of products are currently under development, and may become commercially available in the future, for the treatment of conditions for which we may attempt to develop product candidates. In particular, there is intense competition in the field of metabolic disorders and glaucoma. Our competitors include larger and better funded pharmaceutical, biopharmaceutical, biotechnological and therapeutics companies. Moreover, we may also compete with universities and other research institutions who may be active in metabolic disorders and glaucoma research and could be in direct competition with us. We also compete with these organizations to recruit management, scientists and clinical development personnel, which could negatively affect our level of expertise and our ability to execute our business plan. We will also face competition in establishing clinical trial sites, enrolling subjects for clinical trials and in identifying and in-licensing new product candidates. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
If we successfully obtain approval for any product candidate, we will face competition based on many different factors, including the safety and effectiveness of our products, the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration, the timing and scope of regulatory approvals for these products, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position. Competing products could present superior treatment alternatives, including by being more effective, safer, more convenient, less expensive or marketed and sold more effectively than any products we may develop. Competitive products may make any products we develop obsolete or noncompetitive before we recover the expense of developing and commercializing our product candidates.
For additional information about our competitors and competitive products, see the section entitled "Competition" in Part I, Item 1 of this Annual Report on Form 10-K.
If we are unable to compete effectively, our opportunity to generate revenue from the sale of our products we may develop, if approved, could be material and adversely affected, which would materially adversely affect our results of operations, financial condition and business.
Even if our current or future product candidates receive marketing approval, they may fail to achieve market acceptance by physicians, patients, third-party payors or others in the medical community necessary for commercial success.
Even if our current or future product candidates receive marketing approval, they may fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. If they do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of our current or future product candidates, if approved for commercial sale, will depend on a number of factors, including but not limited to:
•the clinical indications for which the product candidate is approved;
•the efficacy and potential advantages compared to alternative treatments and therapies;
•the timing of market introduction of the product as well as competitive products;
•effectiveness of sales and marketing efforts;
•the strength of our relationships with patient communities;
•the cost of treatment in relation to alternative treatments and therapies, including any similar generic treatments;
•our ability to offer such product for sale at competitive prices;
•the convenience and ease of administration compared to alternative treatments and therapies;
•the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
•the availability of third-party coverage and adequate reimbursement;
•the willingness of patients to pay out-of-pocket in the absence of coverage and adequate reimbursement by third-party payors and government authorities;
•the strength of marketing and distribution support;
33
•the prevalence and severity of any side effects; and
•any restrictions on the use of the product together with other medications.
Our efforts to educate physicians, patients, third-party payors and others in the medical community on the benefits of our product candidates may require significant resources and may never be successful. Such efforts may require more resources than are typically required due to the complexity and uniqueness of our product candidates. Because we expect sales of our product candidates, if approved, to generate substantially all of our revenues for the foreseeable future, the failure of our product candidates, if approved, to find market acceptance would harm our business and could require us to seek additional financing.
Coverage and adequate reimbursement may not be available for our current or any future product candidates, which could make it difficult for us to sell profitably, if approved.
Market acceptance and sales of any product candidates that we commercialize, if approved, will depend in part on the extent to which coverage and adequate reimbursement for these drugs and related treatments will be available from third-party payors, including government health administration authorities, managed care organizations and other private health insurers. Third-party payors decide which therapies they will pay for and establish reimbursement levels. Commercial payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for any product candidates that we develop will be made on a payor-by-payor basis. One third-party payor’s determination to provide coverage for a drug does not assure that other payors will also provide coverage, and adequate reimbursement, for the drug. Additionally, a third-party payor’s decision to provide coverage for a therapy does not imply that an adequate reimbursement rate will be approved. Each third-party payor determines whether or not it will provide coverage for a therapy, what amount it will pay the manufacturer for the therapy, and on what tier of its formulary it will be placed. The position on a third-party payor’s list of covered drugs, or formulary, generally determines the co-payment that a patient will need to make to obtain the therapy and can strongly influence the adoption of such therapy by patients and physicians. Patients who are prescribed treatments for their conditions and providers prescribing such services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our drugs unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our drugs.
A primary trend in the U.S. healthcare industry, and elsewhere, is cost containment. Third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that coverage and reimbursement will be available for any drug that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Inadequate coverage and reimbursement may impact the demand for, or the price of, any drug for which we obtain marketing approval. If coverage and adequate reimbursement are not available, or are available only to limited levels, we may not be able to successfully commercialize our current and any future product candidates that we develop, which could have an adverse effect on our operating results and our overall financial condition. Further, coverage policies and third-party payor reimbursement rates may change at any time. Therefore, even if favorable coverage and reimbursement status is attained for one or more products for which we receive marketing approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
If the market opportunities for any of our product candidates are smaller than we estimate, even assuming approval of a product candidate, our revenue may be adversely affected, and our business may suffer.
The precise incidence and prevalence for all the conditions we aim to address with our product candidates are unknown. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations or market research, and may prove to be incorrect. Further, new information may change the estimated incidence or prevalence of these diseases. The total addressable market across all of our product candidates will ultimately depend upon, among other things, the diagnosis criteria included in the final label for each of our product candidates approved for sale for these indications, the availability of alternative treatments and the safety, convenience, cost and efficacy of our product candidates relative to such alternative treatments, acceptance by the medical community and patient access, drug pricing and reimbursement. The number of patients in the United States and other major markets and elsewhere may turn out to be lower than expected, patients may not be otherwise amenable to treatment with our products or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business.
34
We currently have no marketing and sales organization and have no experience as a company in commercializing products, and we may invest significant resources to develop these capabilities. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our products, we may not be able to generate product revenue.
We have no internal sales, marketing or distribution capabilities, nor have we as a company commercialized a product. If any of our product candidates ultimately receive marketing approval, we will be required to build a marketing and sales organization with technical expertise and supporting distribution capabilities to commercialize each such product in the markets that we target, which will be expensive and time consuming, or collaborate with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. We have no prior experience as a company in the marketing, sale and distribution of biopharmaceutical products and there are significant risks and costs involved in building and managing a sales organization, including our ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training (e.g., about our products and compliance with applicable laws) to sales and marketing personnel and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities or implementation of adequate controls and monitoring to ensure that our sales and marketing activities are in compliance with applicable laws would adversely impact the commercialization of these products. We may not be able to enter into collaborations or hire consultants or external service providers to assist us in sales, marketing and distribution functions on acceptable financial terms, or at all. In addition, our product revenues and our profitability, if any, may be lower if we rely on third parties for these functions than if we were to market, sell and distribute any products that we develop ourselves. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively or in compliance with applicable laws. If we are not successful in commercializing our products, either on our own or through arrangements with one or more third parties, we may not be able to generate any future product revenue and we would incur significant additional losses.
Our future growth may depend, in part, on our ability to commercialize products in foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future growth may depend, in part, on our ability to develop and commercialize our product candidates in foreign markets. We are not permitted to market or promote any of our product candidates before we receive regulatory approval from applicable regulatory authorities in foreign markets, and we may never receive such regulatory approvals for any of our product candidates. To obtain separate regulatory approval in many other countries we must comply with numerous and varying regulatory requirements regarding safety and efficacy and governing, among other things, clinical trials, commercial sales, pricing and distribution of our product candidates. If we obtain regulatory approval of our product candidates and ultimately commercialize our products in foreign markets, we would be subject to additional risks and uncertainties, including:
•different regulatory requirements for approval of drugs in foreign countries;
•reduced protection for intellectual property rights;
•the existence of additional third-party patent rights of potential relevance to our business;
•unexpected changes in tariffs, trade barriers and regulatory requirements;
•economic weakness, including inflation, or political instability in particular foreign economies and markets;
•compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
•foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country;
•foreign reimbursement, pricing and insurance regimes;
•workforce uncertainty in countries where labor unrest is common;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
•business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires.
35
Risks Related to Our Business Operations and Industry
If we are not able to attract and retain highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive biotechnology and pharmaceuticals industries depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. Our success depends in large measure on our key personnel, including Mr. Punit Dhillon, our Chair and Chief Executive Officer, Ms. Kaitlyn Arsenault, our Chief Financial Officer, Mr. Tuan Tu Diep, our Chief Development Officer and Dr. Christopher Twitty, our Chief Scientific Officer as well as other members of our senior management team. The loss of the services of any of these individuals could significantly hinder our operations. We do not currently have "key person" insurance in effect for Mr. Dhillon, Ms. Arsenault, Mr. Diep, Dr. Twitty or other members of our senior management team. In addition, the competition for qualified personnel in the pharmaceutical industry is intense and there can be no assurance that we will be able to continue to attract and retain all personnel necessary for the development and operation of our business. We also rely on, and have relied on in the past, consultants and advisors to assist us in formulating our strategy. Our consultants and advisors are either self-employed or employed by other organizations, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to contribute to us.
Our future performance will also depend, in part, on our ability to successfully integrate newly hired executive officers into our management team and our ability to develop an effective working relationship among senior management. Our failure to integrate these individuals and create effective working relationships among them and other members of management could result in inefficiencies in the development and commercialization of our product candidates, harming future marketing approvals, sales of our product candidates and our results of operations.
Recently enacted legislation, future legislation and healthcare reform measures may increase the difficulty and cost for us to obtain marketing approval for and commercialize our product candidates and may affect the prices we may set.
In the United States and some foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system, including cost-containment measures that may reduce or limit coverage and reimbursement for newly approved drugs and affect our ability to profitably sell any product candidates for which we obtain marketing approval. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs and improve the quality of healthcare.
For example, in March 2010, the ACA was enacted in the United States. Among the provisions of the ACA of importance to our potential product candidates, the ACA: established an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents; expanded eligibility criteria for Medicaid programs; increased the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; created a new Medicare Part D coverage gap discount program; established a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with funding for such research; and established a Center for Medicare Innovation at the Centers for Medicare and Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending.
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how any such challenges and the healthcare reform measures of the Biden administration, or any future presidential administration, will impact the ACA or our business.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, included reductions to Medicare payments to providers, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2032, unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. In addition, on March 11, 2021, the American Rescue Plan Act of 2021 was signed into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, or AMP, beginning January 1, 2024.
36
Further, there has been heightened governmental scrutiny in the United States of pharmaceutical pricing practices in light of the rising cost of prescription drugs. Such scrutiny has resulted in several recent congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. On August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. On June 30, 2023 the Centers for Medicare and Medicaid Services, or CMS, issued new guidance detailing the requirements and parameters of the first round of price negotiations, to take place during 2023 and 2024, for products subject to the “maximum fair price” provision that would become effective in 2026. On August 29, 2023, HHS announced the list of the first ten drugs that will be subject to price negotiations, although the Medicare drug price negotiation program is currently subject to legal challenges. CMS and HHS will continue to issue and update guidance as these programs are implemented. For that and other reasons, it is currently unclear how the IRA will be effectuated.
At the state level, individual states in the United States are also increasingly active in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including prescription drug affordability boards, price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our product candidates, if approved, or put pressure on our product pricing, which could negatively affect our business, results of operations, financial condition and prospects.
We expect that these new laws and other healthcare reform measures that may be adopted in the future may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our product candidates, if approved, which could have a material adverse effect on our results of operations and financial condition.
If product liability or state consumer protection act lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products.
We face an inherent risk of product liability as a result of the clinical trials of our product candidates and will face an even greater risk if we commercialize our product candidates. For example, we may be sued if our product candidates allegedly cause injury or are found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product candidate, negligence, strict liability and a breach of warranties. Claims may be brought against us by clinical trial participants, patients or others using, administering or selling products that may be approved in the future, and could be asserted as product liability claims or under state consumer protection acts.
If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit or cease the commercialization of our products. Even a successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
•decreased demand for our products;
•injury to our reputation and significant negative media attention;
•withdrawal of clinical trial participants and potential termination of clinical trial sites or entire clinical programs;
•costs to defend the related litigation;
•a diversion of management’s time and our resources;
•substantial monetary awards to trial participants or patients;
•product recalls, withdrawals or labeling, marketing or promotional restrictions;
37
•initiation of investigations and enforcement actions by regulators;
•significant negative financial impact;
•the inability to commercialize our product candidates; and
•a decline in our stock price.
We currently hold $3,000,000 in product liability insurance coverage in the aggregate. We may need to increase our insurance coverage as we expand our clinical trials or if we commence commercialization of our product candidates. Insurance coverage is increasingly expensive. Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of our product candidates. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies will also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We may have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts, which could have a material adverse effect on our business, results of operations and financial condition.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for any of our product candidates and begin commercializing those products in the United States, our operations may be directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician sunshine laws and regulations. These laws may impact, among other things, our proposed sales, marketing, and education programs. In addition, we may be subject to privacy and security obligations, including federal and state laws, regulations, guidance, and industry standards related to data privacy, security, and protection. The laws that may affect our ability to operate include:
•the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a government healthcare program, such as the Medicare and Medicaid programs;
•federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third party payors that are false or fraudulent;
•HIPAA, which imposes certain requirements relating to the privacy, security, and transmission of PHI;
•the federal physician sunshine requirements under the ACA, which require manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members; and
•state law equivalents of each of the above federal laws, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities, or those of our third-party collaborators or service providers, could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. If our operations, or those of our third-party collaborators or service providers, are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
38
Our business and operations would be adversely affected in the event that our computer systems or those of our partners, contract research organizations, contractors, consultants or other third parties we work with were to suffer system failures, cyber-attacks, loss of data or other security incidents.
Despite our efforts to implement security measures on our computer systems, as well as those of our partners, contract research organizations, contractors, consultants, law and accounting firms and other third parties we work with, may sustain damage from security vulnerabilities, unauthorized access, data breaches, phishing attacks, ransomware attacks, denial-of-service attacks, cybercriminals, natural disasters, terrorism, war and telecommunication and electrical failures. We rely on our partners and third-party providers to implement effective security measures and identify and correct for any such failures, deficiencies or breaches. The risks of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, nation state actors and cyber-terrorists, have increased significantly and are becoming increasingly difficult to detect. If a cybersecurity-related incident or security breach were to occur, it may cause interruptions in our operations, or the operations of our partners or third-party providers, it could result in a the misuse of sensitive information, including personal data (including health data), our intellectual property or financial information, trade secrets, or clinical trial participant personal data, a material disruption or delay in our drug development programs, and/or significant monetary losses. For example, during the second quarter of 2022, we were indirectly impacted by a cyberattack on our Phase 1 clinical supply contract manufacturer which delayed our production timeline and the initiation of enrollment in our Phase 1 clinical studies for SBI-100 OE to the fourth quarter of 2022. Unauthorized access, compromise, damage to, or loss of preclinical or clinical trial data from completed, ongoing or planned trials, or chemistry, manufacturing and controls data for our product candidates, could result in delays in regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Any such breach, loss or compromise of clinical trial participant personal data may also subject us to civil fines and penalties under the privacy laws of the European Union or other countries as well as state and federal privacy laws in the United States. We maintain cyber liability insurance; however, this insurance may only partly cover the financial, legal, business or reputational losses that may result from an interruption or breach of our systems or the systems of our service providers.
Actual or perceived failures to comply with applicable data protection, privacy and security laws, regulations, standards and other requirements could have a material adverse effect on our business, financial condition or results of operations.
Privacy and data security have become a significant area of focus in the U.S., and in many other jurisdictions where we may in the future conduct our operations. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues, which may affect our business and may increase our compliance costs and exposure to liability. As we receive, collect, process, use and store personal and confidential data, we are or may be subject to multiple laws and regulations relating to data privacy and security. Compliance with these privacy and data security requirements is rigorous and time-intensive and may increase our cost of doing business, and despite those efforts, there is a risk that we may be subject to fines and penalties, litigation and reputational harm, which could materially and adversely affect our business, financial condition and operations.
In the U.S., we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA imposes, among other things, requirements relating to the privacy, security, transmission and breach reporting of PHI held by covered entities and their business associates. We may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under HIPAA. Depending on the facts and circumstances, we could be subject to criminal penalties if we knowingly receive individually identifiable health information from a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
39
In addition, state laws govern the privacy and security of health-related and other personal information in certain circumstances, many of which differ from each other in significant ways and may not have the same requirements, thus complicating compliance efforts. Several states, including California, Colorado, Connecticut, Utah and Virginia, have adopted generally applicable and comprehensive privacy laws, although most have an exception for information regulated by HIPAA. These aws provide a number of individual privacy rights and impose corresponding obligations on organizations doing business in these states. By way of example, California enacted the California Consumer Privacy Act (“CCPA”), effective January 1, 2020 and amended by the California Privacy Rights Act, effective January 1, 2023, which imposes obligations on covered businesses to provide specific disclosures related to a business’s collecting, using, and disclosing personal data and to respond to certain requests from California residents related to their personal data. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that has increased the likelihood of, and risks associated with, data breach litigation. The CCPA may increase our compliance costs and potential liability. It also created a new California data protection agency, the California Privacy Protection Agency, which is authorized to issue substantive regulations and could result in increased privacy and information security enforcement and additional compliance investment and potential business process changes may be required Similar laws have passed in Colorado, Connecticut, Delaware, Indiana, Iowa, Montana, Oregon, Tennessee, Texas, Utah, and Virginia and have been proposed in other states and at the federal level, reflecting a trend toward more stringent privacy legislation in the United States. Further states have also enacted consumer health data privacy laws, including states without comprehensive consumer privacy laws, such as Nevada and Washington state. Such laws could have different requirements that would make compliance challenging. In the event that we are subject to HIPAA, the CCPA, the CPRA or other privacy and data protection laws, any liability from failure to comply with the requirements of these laws could adversely affect our financial condition as a result of fines, penalties, litigation or other liabilities.
Our employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants and commercial partners. Misconduct by these parties could include intentional failures to comply with FDA regulations or the regulations applicable in other jurisdictions, provide accurate information to the FDA and applicable foreign authorities, comply with healthcare fraud and abuse laws, and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us or comply with other applicable law. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct also could involve the improper use of information obtained in the course of clinical trials or interactions with the FDA or applicable foreign authorities, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could have a negative impact on our business, financial condition, results of operations and prospects, including the imposition of significant fines or other sanctions.
40
The Company is currently subject to lawsuits, and in the future may be subject to additional lawsuits, that could divert its resources and result in the payment of significant damages and other remedies.
From time to time, the Company may be subject to litigation claims through the ordinary course of its business operations or otherwise, regarding, among other things, intellectual property rights matters, employment matters and tax matters. Litigation to defend the Company against claims by third parties, or to enforce any rights that the Company may have against third parties, may be necessary, which could result in substantial costs and diversion of the Company's resources, causing a material adverse effect on its business, financial condition and results of operations. Given the nature of the Company's business, it is, and may from time to time in the future be, party to various, and at times numerous, legal, administrative and regulatory inquiries, investigations, proceedings and claims that arise in the ordinary course of business, as well as potential class action lawsuits. Because the outcome of such legal matters is inherently uncertain, if one or more of such legal matters were to be resolved against the Company for amounts in excess of management's expectations or any applicable insurance coverage or indemnification right, the Company's results of operations and financial condition could be materially adversely affected. Any litigation to which the Company is a party may result in an onerous or unfavorable judgment that may not be reversed upon appeal, or in payments of substantial monetary damages or fines, the posting of bonds requiring significant collateral, letters of credit or similar instruments, or the Company may decide to settle lawsuits on similarly unfavorable terms. Moreover, the Company cannot be sure that the remedies available to it at law or under contract, will be sufficient in amount, scope or duration to fully or partially offset any such possible liabilities. Any of these factors, individually or in the aggregate, could have a material adverse effect on the Company's business, results of operations, cash flows or liquidity. For a description of certain currently pending legal and regulatory proceedings, including the Cunning Lawsuit, see Note 13 to the Notes to the Consolidated Financial Statements of the Company included in Part IV, Item 15 of this Annual Report on Form 10-K.
The increasing use of social media platforms presents new risks and challenges.
Social media is increasingly being used to communicate about our product candidates, technologies and programs, and the diseases our product candidates are designed to treat. Social media practices in the biopharmaceutical industry continue to evolve and regulations relating to such use are not always clear. This evolution creates uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients may use social media channels to comment on the effectiveness of a product candidate or to report an alleged adverse event. When such disclosures occur, there is a risk that we fail to monitor and comply with applicable adverse event reporting obligations or we may not be able to defend ourselves or the public’s legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about our product candidates. There is also a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on any social networking website. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face overly restrictive regulatory actions or incur other harm to our business.
Risks Related to Our Intellectual Property
Our success depends on our ability to protect our intellectual property and our proprietary technologies.
Our commercial success depends in part on our ability to obtain and maintain patent protection and trade secret protection for our product candidates, proprietary technologies and their uses as well as our ability to operate without infringing upon the proprietary rights of others. We generally seek to protect our proprietary position by filing patent applications in the United States and abroad related to our product candidates, proprietary technologies and their uses that are important to our business. We also seek to protect our proprietary position by acquiring or in-licensing relevant issued patents or pending applications, or other intellectual property rights, from third parties.
Pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless, and until, patents issue from such applications, and then only to the extent the issued claims cover the technology and/or its use. There can be no assurance that any of our future patent applications or the patent applications of our licensors will result in additional patents being issued or that issued patents will afford sufficient protection against competitors with similar technology, nor can there be any assurance that the patents issued will not be infringed, designed around or invalidated by third parties.
Even issued patents may later be found invalid or unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for our and our licensors proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep any competitive advantage. These uncertainties and/or limitations in our ability to properly protect the intellectual property rights relating to our product candidates could have a material adverse effect on our financial condition and results of operations.
41
The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that we or any of our potential future collaborators will be successful in protecting our product candidates by obtaining and defending patents.
The patent prosecution process is also expensive and time-consuming, and we and our licensors, such as UM, may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner or in all jurisdictions where protection may be commercially advantageous. It is also possible that we or our licensors will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
In addition, although we enter into non-disclosure and confidentiality agreements with parties who have access to patentable aspects of our research and development output, such as our employees, outside scientific collaborators, contract research organizations, third-party manufacturers, consultants, advisors and other third parties, any of these parties may breach such agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection.
Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
In some circumstances, we may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology or products that we license from third parties. Therefore, we cannot be certain that these patents and applications will be prosecuted and enforced in a manner consistent with the best interests of our business. In addition, if third parties who license patents to us fail to maintain such patents, or lose rights to those patents, the rights we have licensed may be reduced or eliminated.
We may be involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and unsuccessful. Further, our issued patents could be found invalid or unenforceable if challenged in court.
Competitors may infringe our intellectual property rights. To prevent infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in a patent infringement proceeding, a court may decide that a patent we own is not valid, is unenforceable and/or is not infringed. If we or any of our potential future collaborators were to initiate legal proceedings against a third party to enforce a patent directed at one of our product candidates, the defendant could counterclaim that our patent is invalid and/or unenforceable in whole or in part. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include an alleged failure to meet any of several statutory requirements, including but not limited to lack of novelty, obviousness, written description or non-enablement. Grounds for an unenforceability assertion could include an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution.
Third parties may also raise similar invalidity claims before the USPTO or patent offices abroad, even outside the context of litigation. Such mechanisms include re-examination, PGR, IPR, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation of, cancellation of or amendment to our patents in such a way that they no longer cover our technology or platform, or any product candidates that we may develop. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. There is also no assurance that there is not prior art of which we are aware, but which we do not believe affects the validity or enforceability of a claim in our patents and patent applications, which may, nonetheless, ultimately be found to affect the validity or enforceability of a patent claim. If a third party were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates or other intellectual property that we may develop. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates. Such a loss of patent protection would have a material adverse impact on our business, financial condition, results of operations and prospects.
42
Even if resolved in our favor, litigation or other legal proceedings relating to our intellectual property rights may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or other legal proceedings relating to our intellectual property rights, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation or other proceedings. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
•others may be able to develop products that are similar to our product candidates but that are not covered by the claims of the patents that we own;
•we might not have been the first to make the inventions covered by the issued patents or patent application that we own;
•we might not have been the first to file patent applications covering certain of our inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
•it is possible that our pending patent applications will not lead to issued patents;
•issued patents that we own may be held invalid or unenforceable, as a result of legal challenges by our competitors;
•our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable; and
•the patents of others may have an adverse effect on our business.
Should any of these events occur, it could significantly harm our business, results of operations and prospects.
Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties. Claims by third parties that we infringe their proprietary rights may result in liability for damages or prevent or delay our developmental and commercialization efforts.
Our commercial success depends in part on avoiding infringement of the patents and proprietary rights of third parties. However, our research, development and commercialization activities may be subject to claims that we infringe or otherwise violate patents or other intellectual property rights owned or controlled by third parties. Other entities may have or obtain patents or proprietary rights that could limit our ability to make, use, sell, offer for sale or import our product candidates and products that may be approved in the future, or impair our competitive position. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biopharmaceutical industry, including patent infringement lawsuits, oppositions, reexaminations, IPR proceedings and PGR proceedings before the USPTO and/or corresponding foreign patent offices. Numerous third-party U.S. and foreign issued patents and pending patent applications exist in the fields in which we are developing product candidates. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates.
43
As the biopharmaceutical industry expands and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties. Because patent applications are maintained as confidential for a certain period of time, until the relevant application is published, we may be unaware of third-party patents that may be infringed by commercialization of any of our product candidates, and we cannot be certain that we were the first to file a patent application related to a product candidate or technology. Moreover, because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates may infringe. In addition, identification of third-party patent rights that may be relevant to our technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. There is also no assurance that there is not prior art of which we are aware, but which we do not believe is relevant to our business, which may, nonetheless, ultimately be found to limit our ability to make, use, sell, offer for sale or import our products that may be approved in the future, or impair our competitive position. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. Any claims of patent infringement asserted by third parties would be time consuming and could:
•result in costly litigation that may cause negative publicity;
•divert the time and attention of our technical personnel and management;
•cause development delays;
•prevent us from commercializing any of our product candidates until the asserted patent expires or is held finally invalid or not infringed in a court of law;
•require us to develop non-infringing technology, which may not be possible on a cost-effective basis;
•subject us to significant liability to third parties; or
•require us to enter into royalty or licensing agreements, which may not be available on commercially reasonable terms, or at all, or which might be non-exclusive, which could result in our competitors gaining access to the same technology.
Although no third party has asserted a claim of patent infringement against us as of the date of this Annual Report on Form 10-K, others may hold proprietary rights that could prevent our product candidates from being marketed once approved. Any patent-related legal action against us claiming damages and seeking to enjoin commercial activities relating to our products or processes could subject us to potential liability for damages, including treble damages if we were determined to willfully infringe, and require us to obtain a license to manufacture or market our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. We cannot predict whether we would prevail in any such actions or that any license required under any of these patents would be made available on commercially acceptable terms, if at all. Moreover, even if we or our future strategic partners were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. In addition, we cannot be certain that we could redesign our product candidates or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could delay or prevent us from developing and commercializing our product candidates, which could harm our business, financial condition and operating results. In addition, intellectual property litigation, regardless of its outcome, may cause negative publicity and could prohibit us from marketing or otherwise commercializing our product candidates and technology.
Parties making claims against us may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have material adverse effect on our ability to raise additional funds or otherwise have a material adverse effect on our business, results of operations, financial condition and prospects.
Intellectual property litigation may lead to unfavorable publicity that harms our reputation and causes the market price of our common shares to decline.
During the course of any intellectual property litigation, there could be public announcements of the initiation of the litigation as well as results of hearings, rulings on motions, and other interim proceedings in the litigation. If securities analysts or investors regard these announcements as negative, the perceived value of our existing products, programs or intellectual property could be diminished. Accordingly, the market price of shares of our common stock may decline. Such announcements could also harm our reputation or the market for our future products, which could have a material adverse effect on our business.
44
Derivation proceedings may be necessary to determine priority of inventions, and an unfavorable outcome may require us to cease using the related technology or to attempt to license rights from the prevailing party.
Derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of derivation proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with such proceedings could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties or enter into development or manufacturing partnerships that would help us bring our product candidates to market.
Changes in U.S. patent law, or laws in other countries or jurisdictions, could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the pharmaceutical industry involve a high degree of technological and legal complexity. Therefore, obtaining and enforcing pharmaceutical patents is costly, time consuming and inherently uncertain. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property and may increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. In addition, Congress or other foreign legislative bodies may pass patent reform legislation that is unfavorable to us.
For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the U.S. federal courts, the USPTO, or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents we might obtain in the future.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may also be subject to claims that former employees or other third parties have an ownership interest in our patents or other intellectual property. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and distraction to management and other employees.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
45
If we do not obtain patent term extension for our product candidates, our business may be materially harmed.
Depending upon the timing, duration and specifics of FDA marketing approval of our product candidates, one or more of our U.S. patents may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. A maximum of one patent may be extended per FDA approved product as compensation for the patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only those claims covering such approved drug product, a method for using it or a method for manufacturing it may be extended. Patent term extension may also be available in certain foreign countries upon regulatory approval of our product candidates. However, we may not be granted an extension because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or restoration or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our revenue could be reduced, possibly materially. Further, if this occurs, our competitors may take advantage of our investment in development and trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case.
We may not be able to protect our intellectual property rights throughout the world.
Patents are of national or regional effect. Filing, prosecuting and defending patents in all countries throughout the world could be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our product candidates, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of many foreign countries do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights. As an example, as of June 2023, European patent applications have the option, upon grant of a patent, of becoming a Unitary Patent which will be subject to the jurisdiction of the Unitary Patent Court, or UPC. Patents granted before the implementation of the UPC will have the option of opting out of the jurisdiction of the UPC and remaining as national patents in the UPC countries. Patents that remain under the jurisdiction of the UPC may be potentially vulnerable to a single UPC-based revocation challenge that, if successful, could invalidate the patent in all countries who ratified the Unitary Patent Court Agreement. The option of a Unitary Patent will be a significant change in European patent practice. As the UPC is a new court system, there is no precedent for the court, increasing the uncertainty. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected.
Further, the standards applied by the USPTO and foreign patent offices in granting patents are not always applied uniformly or predictably. As such, we do not know the degree of future protection that we will have on our product candidates, proprietary technologies, and their uses. While we will endeavor to try to protect our product candidates, proprietary technologies, and their uses, with intellectual property rights such as patents, as appropriate, the process of obtaining patents is time consuming, expensive, and unpredictable.
46
Further, geo-political actions in the United States and in foreign countries could increase the uncertainties and costs surrounding the prosecution or maintenance of our patent applications or those of any current or future licensors and the maintenance, enforcement or defense of our issued patents or those of any current or future licensors. Accordingly, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by regulations and governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to the USPTO and various foreign patent offices at various points over the lifetime of our patents and/or applications. We have systems in place to remind us to pay these fees, and we rely on our outside patent annuity service to pay these fees when due. Additionally, the USPTO and various foreign patent offices, require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with rules applicable to the particular jurisdiction. However, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If such an event were to occur, it could have a material adverse effect on our business.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition, we rely on the protection of our trade secrets, including unpatented know-how, technology and other proprietary information to maintain our competitive position. Although we have taken steps to protect our trade secrets and unpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements with employees, consultants and advisors, we cannot provide any assurances that all such agreements have been duly executed, and any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets.
Moreover, third parties may still obtain this information or may come upon this or similar information independently, and we would have no right to prevent them from using that technology or information to compete with us. If any of these events occurs or if we otherwise lose protection for our trade secrets, the value of this information may be greatly reduced, and our competitive position would be harmed. If we do not apply for patent protection prior to such publication or if we cannot otherwise maintain the confidentiality of our proprietary technology and other confidential information, then our ability to obtain patent protection or to protect our trade secret information may be jeopardized.
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is common in the pharmaceutical industry, in addition to our employees, we engage the services of consultants to assist us in the development of our product candidates. Many of these consultants, and many of our employees, were previously employed at, or may have previously provided or may be currently providing consulting services to, other pharmaceutical companies including our competitors or potential competitors. We may become subject to claims that we, our employees or a consultant inadvertently or otherwise used or disclosed trade secrets or other information proprietary to their former employers or their former or current clients. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely affect our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management team and other employees.
UM is the owner of intellectual property underlying SBI-100 OE
Intellectual property rights (including any patents, non-manufacturing related know-how and improvements) for SBI-100 is owned by UM, and in the future we may need to seek UM’s consent to pursue, use, sub-license and/or enforce some of these intellectual property rights which we are entitled to use pursuant to the UM 5050 license agreement. An unexpected deterioration in our relationship with UM may have a material adverse effect on our business, reputation, results of operations and financial condition.
47
Breach of the License Agreement with UM could result in the loss of such license rights that are important to our business and our operations could be materially harmed.
We license from UM the use, development and commercialization rights for SBI-100. As a result, our current business plans are dependent upon our maintenance of the UM 5050 license agreement and the rights we license under them. If we breach the terms of our License Agreement with UM, or any future license agreement on which our business or product candidates are dependent, UM or other licensors may have the right to terminate the applicable agreement in whole or in part and thereby limit or terminate our rights to the licensed technology and intellectual property and/or any rights we have acquired to develop and commercialize certain product candidates or cause us to have to negotiate new or reinstated licenses on less favorable terms, or enable a competitor to gain access to the licensed technology. Moreover, disputes may arise regarding intellectual property subject to a license agreement such as our license agreements with UM, including: (i) the scope of the rights granted under the license agreement and other interpretation related issues, (ii) the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement, (iii) our diligence obligations under the license agreement and what activities satisfy those diligence obligations. The loss of the rights licensed to us under our License Agreements with UM, or any future license agreement that we may enter granting rights on which our business or product candidates are dependent, would harm, or even eliminate, our ability to further develop the applicable product candidates and would materially harm our business, prospects, financial condition and results of operations.
Risks Related to Controlled Substances
Our product candidate, SBI-100 OE, will be subject to U.S. controlled substance laws and regulations, and failure to comply with or the cost of compliance with these laws and regulations, may adversely affect the results of our business operations, and our financial condition.
Our product candidate, SBI-100 OE, contains a controlled substance as defined in the CSA. Controlled substances that are pharmaceutical products are subject to a high degree of regulation under the CSA, which establishes, among other things, certain registration, manufacturing quotas, security, recordkeeping, reporting, import, export and other requirements administered by the DEA. The DEA classifies controlled substances into five schedules: Schedule I, II, III, IV or V substances. Schedule I substances by definition have a high potential for abuse, no currently “accepted medical use” in the United States, lack accepted safety for use under medical supervision, and may not be prescribed, marketed or sold in the United States. Pharmaceutical products approved for use in the United States may be listed as Schedule II, III, IV or V. Schedule I and II drugs are subject to the strictest controls under the CSA, including manufacturing and procurement quotas, security requirements and criteria for importation. In addition, dispensing of Schedule II drugs is further restricted. While certain cannabinoids may be classified as Schedule I controlled substances, products approved for medical use in the United States that contain certain cannabinoids must be placed on Schedules II-V, since approval by the FDA satisfies the “accepted medical use” requirement. SBI-100 remains a Schedule I controlled substance, pending a request to re-schedule SBI-100 after marketing authorization by the FDA.
If approved by the FDA, we expect the finished dosage forms of SBI-100 OE to be reevaluated by the DEA and no longer listed as a Schedule I drug. Consequently, SBI-100's manufacture, importation, exportation, domestic distribution, storage, sale and legitimate use may be subject to a significant degree of regulation by the DEA, if the finished dosage form is determined to be a Schedule II drug. In addition, the scheduling process may take one or more years, thereby delaying the launch of the drug product in the United States. Furthermore, if the FDA, DEA, or any foreign regulatory authority determines that any of our drug product candidates may have potential for abuse, it may require us to generate more clinical or other data than we currently anticipate establishing whether or to what extent the substance has an abuse potential, which could increase the cost and/or delay the launch of the drug product.
48
Facilities conducting research, manufacturing, distributing, importing or exporting, or dispensing controlled substances must be registered (licensed) to perform these activities and have the security, control, recordkeeping, reporting and inventory mechanisms required by the DEA to prevent drug loss and diversion. All these facilities must renew their registrations annually, except dispensing facilities, which must renew every three years. The DEA conducts periodic inspections of certain registered establishments that handle controlled substances. Obtaining the necessary registrations may result in delay of the manufacturing, development, or distribution of our product candidates. Furthermore, failure to maintain compliance with the CSA, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, financial condition and results of operations. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to restrict, suspend or revoke those registrations. In certain circumstances, violations could lead to criminal proceedings. Individual states may also establish controlled substance laws and regulations that may require additional regulatory approvals to conduct research and clinical trials in that state. As a result, we or our partners or clinical sites may also be required to obtain separate state registrations, permits or licenses in order to be able to receive, handle, and distribute controlled substances for clinical trials. Delay in obtaining these state registrations, permits or licenses may delay the start of our clinical trials for such products, including SBI-100 OE. While some states automatically schedule a drug based on federal action, other states schedule drugs through rule making or a legislative action. State scheduling may delay commercial sale of any product for which we obtain federal regulatory approval and adverse scheduling could have a material adverse effect on the commercial attractiveness of such product. We or our partners or clinical sites must also obtain separate state registrations, permits or licenses to be able to obtain, handle, and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions by the states in addition to those from the DEA or otherwise arising under federal law.
To conduct clinical trials with SBI-100 OE in the United States, prior to approval, each of our research sites must obtain and maintain a DEA researcher registration that will allow those sites to handle and dispense the product candidate and to obtain the product. If the DEA delays or denies the grant of a research registration to one or more research sites, the clinical trial could be significantly delayed, and we could lose clinical trial sites.
Manufacturing of SBI-100 OE is, and, if approved, will be, subject to the DEA’s annual manufacturing and procurement quota requirements, if classified as Schedule II. The annual quota allocated to us or our contract manufacturers for the controlled substances in our product candidates may not be sufficient to meet commercial demand or complete clinical trials. Consequently, any delay or refusal by the DEA in establishing our, or our contract manufacturers’, procurement and/or production quota for controlled substances could delay or stop our clinical trials or product launches, which could have a material adverse effect on our business, financial position and operations.
If, upon approval of SBI-100 OE, the product is scheduled as Schedule II or III, we would also need to identify wholesale distributors with the appropriate DEA registrations and authority to distribute the product to pharmacies and other health care providers. The failure to obtain, or delay in obtaining, or the loss of any of those registrations could result in increased costs to us. Furthermore, state and federal enforcement actions, regulatory requirements, and legislation intended to reduce prescription drug abuse, such as the requirement that physicians consult a state prescription drug monitoring program may make physicians less willing to prescribe, and pharmacies to dispense, our products, if approved.
Research restrictions, product shipment delays or prohibitions could have a material adverse effect on our business, results of operations and financial condition.
Research on and the shipment, import and export of SBI-100 OE and the API used in SBI-100 OE will require research permits, import and export licenses by many different authorities. For instance, in the United States, the FDA, U.S. Customs and Border Protection, and the DEA; in Canada, the Canada Border Services Agency, and Health Canada; in Europe, the European Medicines Agency and the European Commission; in Australia and New Zealand, the Australian Customs and Border Protection Service, the Therapeutic Goods Administration, the New Zealand Medicines and Medical Device Safety Authority and the New Zealand Customs Service; and in other countries, similar regulatory authorities, regulate the research on and import and export of pharmaceutical products that contain controlled substances, such as SBI-100 OE. Specifically, the import and export process requires the issuance of import and export licenses by the relevant controlled substance authority in both the importing and exporting country. We may not be granted, or if granted, maintain, such licenses from the authorities in certain countries. Even if we obtain the relevant licenses, shipments of API and our product candidates may be held up in transit, which could cause significant delays and may lead to product batches being stored outside required temperature ranges. Inappropriate storage may damage the product shipment resulting in delays in clinical trials. Once shipment is complete, we or the research contractors we are working with may also suffer further delays or restrictions as a result of regulations governing research on controlled substances. A delay in a clinical trial or, upon commercialization, a partial or total loss of revenue from one or more shipments of API or our product candidates could have a material adverse effect on our business, results of operations and financial condition. The aforementioned examples and lists of various authorities that may currently, or in the future, affect our ability to conduct research on or import or export our product candidates and/or API, should not be construed as exhaustive or comprehensive in any way.
49
Laws and regulations affecting therapeutic uses of cannabinoids are constantly evolving.
The constant evolution of laws and regulations affecting the research and development of cannabinoid-based pharmaceutical products and treatments could detrimentally affect our business. Laws and regulations related to the therapeutic uses of cannabinoids are subject to changing interpretations. These changes may require us to incur substantial costs associated with legal and compliance fees and ultimately require us to alter our business plan. Furthermore, violations or alleged violation of these laws could disrupt our business and result in a material adverse effect on our operations. In addition, we cannot predict the nature of any future laws, regulations, interpretations or applications of laws and regulations and it is possible that new laws and regulations may be enacted in the future that will be directly applicable to our business.
SBI-100 OE and our potential future product candidates may contain controlled substances, the use of which may generate public controversy
Since SBI-100 OE and our potential product candidates may contain controlled substances, their regulatory approval may generate public controversy or scrutiny. Political and social pressures and adverse publicity could lead to delays in approval of, and increased expenses for, our product candidates. These pressures could also limit or restrict the introduction and marketing of our product candidates. Adverse publicity from misuse or adverse side effects cannabinoid derivatives may adversely affect the commercial success or market penetration achievable by our product candidates. The nature of our business will likely attract a high-level of public and media interest, and in the event of any resultant adverse publicity, our reputation may be harmed.
Risks Related to Our Common Stock
The trading price of our common stock has been volatile with substantial price fluctuations on heavy volume, which could result in substantial losses for purchasers of our common stock and existing stockholders.
Our stock price has been and, in the future, may be subject to substantial volatility. During the fiscal year ended December 31, 2023 through March 20, 2024, the price per share of our common stock has ranged as low as $1.44 and as high as $18.00.
Furthermore, the stock market in general and the market for biopharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above the price paid for the shares. The market price for our common stock may be influenced by many factors, including:
•announcements relating to development, regulatory approvals or commercialization of our product candidates or those of competitors;
•results of clinical trials of our product candidates or those of our competitors;
•announcements by us or our competitors of significant strategic partnerships or collaborations or terminations of such arrangements;
•actual or anticipated variations in our operating results and whether we have achieved key business targets;
•sales of our common stock, including sales by our directors and officers or specific stockholders;
•changes in, or our failure to meet, financial estimates by us or by any securities analysts who might cover our stock;
•changes in securities analysts’ buy and/or sell recommendations;
•general economic, political, or stock market conditions;
•conditions or trends in our industry;
•changes in laws or other regulatory actions affecting us or our industry;
•stock market price and volume fluctuations of comparable companies and, in particular, those that operate in the biopharmaceutical industry;
•announcements of investigations or regulatory scrutiny of our operations or lawsuits filed against us;
•capital commitments;
•investors’ general perception of our company, our business, and our prospects;
•disputes concerning our intellectual property or other proprietary rights; and
•recruitment or departure of key personnel.
50
In the past, stockholders have initiated class action lawsuits against pharmaceutical and biotechnology companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources from our business.
We effected the Reverse Stock Split on September 8, 2023 and the liquidity of our common stock may be continue to be adversely effected.
On September 6, 2023, we filed a Certificate of Change and Certificate of Correction with the Secretary of State of the State of Nevada, which effected a reverse stock split (the “Reverse Split”), at a ratio of one-for-250, of the Company’s issued and outstanding shares of common stock, par value $0.001 per share (the “Common Stock”). The Reverse Split became effective on September 8, 2023. The liquidity of the shares of our common stock may continue to be affected adversely by the Reverse Stock Split given the reduced number of shares of our common stock that are outstanding following the Reverse Stock Split, particularly if the market price of our common stock does not increase from its recent decline partly as a result of the Reverse Stock Split. Following the Reverse Stock Split, the market price of our common stock may not attract new investors and may not satisfy the investing requirements of those investors. There can be no assurance that our share prices will attract new investors, including institutional investors. In addition, there can be no assurance that the market price of our common stock will satisfy the investing requirements of those investors. As a result, the trading liquidity of our common stock may not necessarily improve.
We have never declared dividends and do not anticipate paying any cash dividends.
We have not declared or paid any dividends on any of our capital stock to date. The payment of dividends, if any, would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any dividends will be within the discretion of our Board. We presently intend to retain all earnings, if any, to implement our business plan; accordingly, we do not anticipate the declaration of any dividends in the foreseeable future. Unless and until we pay dividends, stockholders may not receive a return on their shares of common stock.
Our executive officers, directors and principal stockholders, if they choose to act together, have the ability to control or significantly influence all matters submitted to stockholders for approval.
Our executive officers, directors and greater than 5% stockholders, in the aggregate, own approximately 64% of our outstanding common stock as of March 20, 2024. As a result, such persons, acting together, have the ability to control or significantly influence all matters submitted to our stockholders for approval, including the election and removal of directors and approval of any significant transaction, as well as our management and business affairs. This concentration of ownership may have the effect of delaying, deferring or preventing a change in control, impeding a merger, consolidation, takeover or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our business, even if such a transaction would benefit other stockholders.
Additionally, under the terms of the securities purchase agreement (the "January PIPE SPA") entered into in connection with the January PIPE Financing, so long as the investors in the January PIPE Financing continue to beneficially own in the aggregate at least 40% of the securities issued in the January PIPE Financing (such securities, the "Closing Securities"), the Company may not transfer, license (other than in the ordinary course of business), encumber, or sell a royalty interest in any intellectual property relating to nimacimab unless Skye obtains the written consent of Qualified Investors that, together with their respective affiliates, beneficially own at least a majority of the then outstanding Closing Securities owned by the Qualified Investors and their respective affiliates. The term "Qualified Investors" means any investor that, together with its affiliates, continues to own at least 80% of the securities originally purchased by it under the January PIPE SPA. This restriction may have the effect of delaying, deferring or preventing a change in control, impeding a merger, consolidation, takeover or other business combination involving us, or discouraging a potential acquiror from making a tender offer or otherwise attempting to obtain control of our business, even if such a transaction would benefit other stockholders.
51
We have a substantial number of authorized common shares available for future issuance that could cause dilution to our Stockholders’ interest and adversely impact the rights of the holders of our Shares.
We have a total of 100,000,000 shares of common stock authorized for issuance and up to 200,000 shares of preferred stock with the rights, preferences and privileges that our Board may determine from time to time. As of March 20, 2024, we have reserved; 1,201,398 shares for issuance upon the exercise of outstanding options, 1,127,777 shares for issuance upon the vesting of outstanding restricted stock units, 127,034 shares for issuance under our equity incentive plan, 112,000 shares for issuance under our 2022 employee stock purchase plan, and 13,259,679 shares for issuance upon the exercise of outstanding warrants. As of March 20, 2024, we had no outstanding preferred stock. As of March 20, 2024, we had 55,140,232 shares of common stock unreserved and available for issuance. We may seek financing that could result in the issuance of additional shares of our capital stock and/or rights to acquire additional shares of our capital stock. We may also make acquisitions that result in issuances of additional shares of our capital stock. Those additional issuances of capital stock would result in a significant reduction of your percentage interest in us. Furthermore, the book value per share of our common stock may be reduced. This reduction would occur if the exercise price of any issued warrants, the conversion price of any convertible notes is lower than the book value per share of our common stock at the time of such exercise or conversion.
The addition of a substantial number of shares of our common stock into the market or by the registration of any of our other securities under the Securities Act of 1933, as amended (the “Securities Act”), may significantly and negatively affect the prevailing market price for our common stock. The future sales of shares of our common stock issuable upon the exercise of outstanding warrants may have a depressive effect on the market price of our common stock, as such warrants would be more likely to be exercised at a time when the price of our common stock is greater than the exercise price.
The issuance of shares upon exercise of outstanding warrants, convertible debt and options may cause immediate and substantial dilution to our existing stockholders.
If the price per share of our common stock at the time of exercise of any warrants, options, or any other convertible securities is in excess of the various conversion or exercise prices of these convertible securities, conversion or exercise of these convertible securities would have a dilutive effect on our common stock. As of March 20, 2024, we had outstanding (i) warrants to purchase up to 13,259,679 shares of our common stock at exercise prices ranging from $0.001 to $1,250 per share, (ii) options to purchase up to 1,201,398 shares of our common stock at exercise prices ranging from $1.69 to $750.00 per share, (iii) 1,127,777 unreleased restricted stock units exchangeable for shares of our common stock upon vesting. Additionally, we have an outstanding convertible secured promissory note in the principal amount of $5,000,000 with a conversion price of $5.16. Further, any additional financing that we secure may require the granting of rights, preferences or privileges senior to those of our common stock and which result in additional dilution of the existing ownership interests of our common stockholders.
Our ability to utilize our net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. In general, an “ownership change” occurs if the aggregate stock ownership of one or more stockholders or groups of stockholders who own at least 5% of a corporation’s stock increase their ownership by more than 50 percentage points over their lowest ownership percentage within a rolling three-year period. Similar rules may apply under state tax laws. If we experience ownership changes as a result of future transactions in our stock, our ability to use our net operating loss carryforwards and other tax attributes to offset U.S. federal taxable income may be subject to further limitations, which could potentially result in increased future tax liability to us.
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market or the perception that these sales might occur could significantly reduce the market price of our common stock and impair our ability to raise adequate capital through the sale of additional equity securities.
The holders of 24,140,023 shares of our outstanding common stock, or approximately 86% of our total outstanding common stock as of March 20, 2024, are entitled to rights with respect to the registration of their shares under the Securities Act. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by affiliates, as defined in Rule 144 under the Securities Act. Sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
52
We cannot assure you that our common stock will become eligible for listing or quotation on any exchange and the failure to do so may adversely affect your ability to dispose of our common stock in a timely fashion.
We have, and may in the future, consider actions that make us eligible to list our common shares on a stock exchange, such as NASDAQ. We may not be able satisfy the initial standards for listing or quotation on any exchange in the foreseeable future or at all. Even if we are able to become listed or quoted on an exchange, we may not be able to maintain a listing of the common stock on such stock exchange.
Our common shares are thinly-traded, and in the future, may continue to be thinly-traded, and you may be unable to sell at or near ask prices or at all.
Our common shares are quoted on the OTCQB and are thinly traded. We cannot predict whether, and the extent to which, an active public market for our common stock will develop or be sustained due to a number of factors, including the fact that we are a small company that is relatively unknown to stock analysts, stock brokers, institutional investors, and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and may be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there have been, and may continue to be, periods of several days or more when trading activity in our shares is minimal or non-existent. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained.
The market for our common shares can be characterized by significant price volatility when compared to other more well known issuers, and we expect that our share price will continue to be more volatile than a well-known issuer for the indefinite future. The volatility in our share price is attributable to a number of factors. First, as noted above, our common shares have been, and may continue to be, sporadically and/or thinly traded. As a consequence of this lack of liquidity, the trading of relatively small quantities of shares by our stockholders may disproportionately influence the price of those shares in either direction. Secondly, an investment in us is a speculative or “risky” investment due to our lack of revenues or profits to date. You should not invest in our common shares unless you have the ability to tolerate a thinly traded and volatile market for the shares.
General Risk Factors
If securities or industry analysts do not publish research or reports or publish unfavorable research or reports about our business, our stock price and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. We currently have limited research coverage by securities and industry analysts. If securities or industry analysts do not continue coverage of our company, the trading price for our stock would be negatively impacted. In the event one or more of the analysts who covers us downgrades our stock, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to regularly publish reports on us, interest in our stock could decrease, which could cause our stock price or trading volume to decline.
We engage in transactions with related parties which present possible conflicts of interest that could have an adverse effect on us.
We have entered, and may continue to enter, into transactions with affiliates and other related parties for financing, corporate, business development and operational services. Such transactions may not have been entered into on an arm’s-length basis, and we may have achieved more or less favorable terms because such transactions were entered into with our related parties. We rely, and will continue to rely, on our related parties to maintain these services. If the pricing for these services changes, or if our related parties cease to provide these services, including by terminating agreements with us, we may be unable to obtain replacements for these services on the same terms without disruption to our business. This could have a material effect on our business, results of operations and financial condition. The details of certain of these transactions are set forth in “Certain Relationships and Related Party Transactions”. Related party transactions create the possibility of conflicts of interest with regard to our management, we may enter into contracts between us, on the one hand, and related parties, on the other, that may not result in arm’s-length transactions, including that:
•our executive officers and directors that hold positions of responsibility with related parties may be aware of certain business opportunities that are appropriate for presentation to us as well as to such other related parties and may present such business opportunities to such other parties; and
•our executive officers and directors that hold positions of responsibility with related parties may have significant duties with, and spend significant time serving, other entities and may have conflicts of interest in allocating time.
53
Such conflicts could cause an individual in our management to seek to advance his or her economic interests or the economic interests of certain related parties above ours. Further, the appearance of conflicts of interest created by related party transactions could impair the confidence of our investors. Our audit committee reviews these transactions. Notwithstanding this, it is possible that a conflict of interest could have a material adverse effect on our liquidity, results of operations and financial condition.
Unpredictable business disruptions could seriously harm our future revenues and financial condition, increase our costs and expenses, and impact our ability to raise capital.
Our operations could be subject to unpredictable events, such as earthquakes, power shortages, telecommunications failures, water shortages, medical epidemics and other natural or man made disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. Notably, we rely on third party manufacturers to produce our product candidates, and such third party manufacturers ability to manufacture our products could be negatively affected by such events.
The current volatility of global financial conditions and inflation could negatively impact our business and financial condition.
Current global financial conditions and recent market events have been characterized by increased volatility, inflation and the resulting tightening of the credit and capital markets has reduced the amount of available liquidity and overall economic activity. Economic factors over which the Company has no control, including changes in inflation, interest rates and foreign currency rates may have a potential adverse effect of on revenues, expenses and resulting margins. We cannot guarantee that debt or equity financing, and the ability to borrow funds or cash generated by operations will be available or sufficient to meet or satisfy our initiatives, objectives, or requirements. Our inability to access sufficient amounts of capital on terms acceptable to us for our operations will negatively impact our business, prospects, liquidity and financial condition.
Global markets have recently experienced increased rates of inflation. Inflation itself, as well as certain governmental efforts to combat inflation, may have significant negative effects on any economy which the Company does business. Past governmental efforts to curb inflation also involved other more drastic economic measures. Any future economic measures to curb inflation could be expected to have similar adverse effects on the level of economic activity in the market, which the Company does business and, in turn, on the operations of the Company. For example, the Federal Reserve recently raised interest rates multiple times in response to concerns about inflation and it may raise them again. Higher interest rates, coupled with reduced government spending and volatility in financial markets may increase economic uncertainty and affect consumer spending. Increased inflation rates can adversely affect us by increasing our costs, including labor and employee benefit costs. Other policies and measures adopted by governments include interest rate adjustments, intervention in the currency markets or actions to adjust or fix the value of the local currency may adversely affect the Company’s business and results of operations.
Adverse U.S. or international economic conditions could negatively affect our business, financial condition and results of operations. We face risks associated with U.S. and international economic conditions and are subject to events beyond our control including war, public health crises, trade disputes, economic sanctions, and their collateral impacts. Adverse U.S. or international economic conditions or periods of inflation or high energy prices may contribute to higher unemployment levels, decreased consumer spending, reduced credit availability and declining consumer confidence and demand, each of which poses a risk to our business. In February 2022, armed conflict escalated between Russia and Ukraine. The sanctions imposed by the U.S. and other countries against Russia, following Russia’s invasion of Ukraine, to date include restrictions on selling or importing goods, services, or technology in or from affected regions and travel bans and asset freezes impacting connected individuals and political, military, business and financial organizations in Russia. The U.S. and other countries could impose wider sanctions and take other actions should the conflict further escalate. It is not possible to predict the broader consequences of this conflict, which could include further sanctions, embargoes, regional instability, geopolitical shifts and adverse effects on macroeconomic conditions, currency exchange rates and financial markets, all of which could impact our business, financial condition and results of operations.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
We maintain a cyber risk management program designed to identify, assess, manage, mitigate, and respond to cybersecurity threats. This program, in conjunction with the Company’s enterprise risk management assessment processes, addresses cybersecurity risks to the corporate information technology (“IT”) environment including systems, hardware, software, data, people, and processes.
54
The underlying processes and controls of our cyber risk management program are designed based on standards for cybersecurity and information technology, including the National Institute of Standards and Technology (“NIST”) Cybersecurity Framework (“CSF”). Skye has an annual assessment performed by a third-party specialist of its cyber risk management program against the NIST CSF. The annual risk assessment identifies, quantifies, and categorizes significant cyber risks. In addition, the Company, in conjunction with the third-party cyber risk management specialists develop a risk mitigation plan to address identified risks and, where necessary, remediate potential issues identified through the annual assessment process.
In addition, we maintain an information security policy that covers safeguarding and managing confidential information, handling personal and company-sensitive data, managing access on/off-boarding and user accounts, acceptable use and IT change management to help govern the processes put in place by management designed to protect Skye’s IT assets, data, and services from threats and vulnerabilities. We partner with industry recognized cybersecurity providers leveraging third-party technology and expertise. We and our cybersecurity partners maintain an IT assets inventory, identity access management controls including restricted access of privileged accounts, physical security measures at Company facilities, information protection/detection systems including maintenance of firewalls and anti-malware tools, network and data traffic monitoring and automated alerting, capacity management, industry-standard encryption protocols, formalized change management processes, critical data backups infrastructure maintenance, incident response, cybersecurity strategy, and cyber risk advisory, assessment and remediation.
Our management team is responsible for oversight and administration of our cyber risk management program, and for informing senior management and other relevant stakeholders regarding the prevention, detection, mitigation, and remediation of cybersecurity incidents. Our management team has experience selecting, deploying, and overseeing cybersecurity technologies, initiatives, and processes directly or via selection of strategic third-party partners, and relies on threat intelligence as well as other information obtained from governmental, public, or private sources, including external consultants engaged by Skye for strategic cyber risk management, advisory and decision making.
We have implemented third-party risk management processes to manage the risks associated with reliance on vendors, critical IT service providers, and other third-parties that may lead to a service disruption or an adverse cybersecurity incident. This includes processes for performing due diligence upon on-boarding.
The Audit Committee of the Board of Directors oversees our cybersecurity risk exposures and the steps taken by management to monitor and mitigate cybersecurity risks. The cybersecurity stakeholders, including management assigned with cybersecurity oversight responsibility and/or third-party consultants providing cyber risk services brief the Audit Committee on cyber vulnerabilities identified through the risk management process, the effectiveness of our cyber risk management program, and the emerging threat landscape and new cyber risks on at least an annual basis. This includes updates on our processes to prevent, detect, and mitigate cybersecurity incidents. In addition, cybersecurity risks are reviewed by our Board of Directors at least annually, as part of our corporate risk oversight processes.
We face risks from cybersecurity threats that could have a material adverse effect on its business, financial condition, results of operations, cash flows or reputation. We acknowledge that the risk of cyber incidents is prevalent in the current threat landscape and that a future cyber incident may occur in the normal course of its business. However, prior cybersecurity incidents have not had a material adverse effect on our business, financial condition, results of operations, or cash flows. We proactively seek to detect and investigate unauthorized attempts and attacks against IT assets, data, and services, and to prevent their occurrence and recurrence where practicable through changes or updates to internal processes and tools and changes or updates to the our service delivery; however, potential vulnerabilities to known or unknown threats will still remain and we may not be able to prevent security incidents in the future. Further, there is increasing regulation regarding responses to cybersecurity incidents, including reporting to regulators, investors, and additional stakeholders, which could subject us to additional liability and reputational harm. In response to such risks, we have implemented initiatives such as implementation of the cybersecurity risk assessment process and development of an incident response plan. See Item 1A. "Risk Factors" for more information on cybersecurity risks.
Item 2. Properties.
We lease our principal executive offices and corporate offices, which are located at 11250 El Camino Real Suite 100, San Diego, CA 92130.
Item 3. Legal Proceedings.
See Note 13 to the accompanying consolidated financial statements included in Part IV, Item 15 of this Annual Report on Form 10-K.
Item 4. Mine Safety Disclosures.
Not applicable.
55
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information.
Our common stock has been quoted on the OTCQB, under the symbol “SKYE”. You should be aware that over-the-counter market quotations may reflect inter-dealer prices, without retail mark-up, mark-down or commissions and may not necessarily represent actual transactions. On March 20, 2024, the last reported sale price of our common stock on the OTCQB was $12.65 per share.
Holders. As of March 20, 2024, there were 109 stockholders of record. The number of stockholders of record does not include beneficial owners of our common stock, whose shares are held in the names of various dealers, clearing agencies, banks, brokers and other fiduciaries.
Dividends. We have never declared or paid a cash dividend on our common stock. We do not expect to pay cash dividends on our common stock in the foreseeable future. We currently intend to retain our earnings, if any, for use in our business. Any dividends declared in the future will be at the discretion of our Board and subject to any restrictions that may be imposed by our lenders.
Performance Graph. We were a smaller reporting company, as defined by Rule 12b-2 of the Securities Exchange Act of 1934, as amended, as of December 31, 2023, and are not required to provide a performance graph.
Recent Sales of Unregistered Securities. None.
Issuer Purchases of Equity Securities. None during the fiscal year ended December 31, 2023 covered by this Annual Report.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements for the years ended December 31, 2023 and 2022 together with notes thereto. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including, but not limited, to those set forth under “Risk Factors” and elsewhere in this Annual Report on Form 10-K.
Unless otherwise provided in this Annual Report, references to “we,” “us,” “our” and “Skye Bioscience” in this discussion and analysis refer to Skye Bioscience, Inc., a Nevada corporation , together with its wholly owned subsidiaries, Nemus, a California corporation, SKYE Bioscience Pty Ltd ("SKYE Bioscience Australia"), an Australian proprietary limited company, Emerald Health Therapeutics, Inc. (EHT) a corporation governed by the Business Corporations Act (British Columbia), Birdrock Bio Sub, Inc. ("BRB"), a Delaware corporation, Ruiyi Acquisition Corp, a Delaware corporation and Avalite Sciences, Inc. (AVI) a corporation governed by the Business Corporations Act (British Columbia).
Overview
We are a clinical stage biopharmaceutical company with a mission to pioneer and lead the development of new pharmaceutical products that unlock the potential of the ECS. Our strategy and clinical assets focus, initially, on the modulation of the CB1 axis to advance the standard of care and provide novel alternative therapies to treat diseases with neuropathic, inflammatory, and metabolic conditions.
Our lead clinical program's product candidate nimacimab, is a peripherally-restricted negative allosteric modulating antibody specific for the human CB1 receptor, administered as a subcutaneous injectable for the treatment of metabolic disorders, including obesity. We plan to launch a Phase 2 clinical trial of nimacimab, which will include a combination study with a GLP-1 agonist, to treat obesity by mid-year 2024, with final data in late 2025.
56
In August of 2023, we acquired nimacimab from the acquisition of BRB as a Phase 2 ready asset. Nimacimab's Phase 1 trial was designed to test the safety and tolerability in a single ascending dose (SAD) in health volunteers and a multiple ascending dose (MAD) in patients with NAFLD. The Phase 1 study, indicated a strong safety profile and provided important indications of effectiveness in reducing cholesterol levels in the NAFLD population. Based on the results of this study, we further evaluated other potential use cases for nimacimab in metabolic, inflammatory and fibrotic processes. Based on our comprehensive review of the overall market, clinical pipeline of competition and potential target product profile (TPP) of nimacimab, in December 2023, we filed an IND to treat patients with obesity in a Phase 2 study.
Our other product candidate, SBI-100 OE, is a Phase 2-stage CB1 agonist (activator) delivered topically into the eye for the treatment of glaucoma and ocular hypertension. Our proprietary eye drop is a nano emulsion formulation that has been developed in a way that provides enhanced bio-availability and permeability, while also extending the duration of activity. In February 2024, we announced the completion of patient enrollment in our Phase 2a placebo controlled study designed to treat glaucoma and ocular hypertension and are expecting to report data in Q2 2024.
We commenced dosing of our Phase 1 clinical study for SBI-100 OE in December 2022 and in November 2023 we reported data demonstrating that SBI-100 OE was safe and well-tolerated. Importantly, we determined that there was minimal systemic exposure of the active metabolite of SBI-100 OE, THC, thus resulting in little to no side effects related to THC intoxication. Moreover, it was determined that after multiple days of dosing we saw minimal hyperaemia (i.e. redness of the eyes) following administration of SBI-100 OE.
In preclinical experiments using SBI-100 OE we have demonstrated statistically superior IOP lowering compared to the prostaglandin-based therapy, latanoprost, the current standard-of-care for treating glaucoma. Statistical significance was reached across multiple time points during a seven-day course of dosing using a validated rabbit normotensive ocular model and SBI-100 exerted pharmacologic activity consistent with once-daily to twice-daily dosing.
We believe that both of our drug candidates are differentiated in their respective markets and target indications with a large unmet need. Because the modulation of the ECS through CB1 has been shown to play a role in both glaucoma and obesity, we believe that both our products are strong candidates for marketing authorization as either first or second-line therapies.
In January 2024 and March 2024, we completed two private placement equity transactions with institutional investors, in which we raised combined net aggregate proceeds of approximately $83,500,000. The capital from the January and March PIPE financings will allow us to fund both of our planned clinical trials for glaucoma and obesity through top line Phase 2 data.
On September 6, 2023, we filed a Certificate of Change and Certificate of Correction with the Secretary of State of the State of Nevada, which effected a reverse stock split, at a ratio of one-for-250, of the Company’s issued and outstanding shares of common ctock (the "Reverse Split"). The Reverse Split was effective on September 8, 2023. As a result of the Reverse Split, each two-hundred fifty (250) shares of common stock was combined into one (1) share of common stock and the total number of shares of common stock authorized was reduced from 5,000,000,000 to 20,000,000 and the number of shares of common stock issued and outstanding was reduced from 3,078,137,871 shares of common stock to 12,312,551 shares of common stock. Subsequently, on November 6, 2023, we increased our authorized shares of common stock to 100,000,000.
On August 18, 2023, we completed a strategic transaction to acquire a clinical asset pursuant to an Agreement and Plan of Merger and Reorganization, dated as of August 15, 2023, by and among the Company, Bird Rock Bio, Inc. and Aquila Merger Sub, Inc., pursuant to which Aquila Merger Sub, Inc. merged with and into Bird Rock Bio, Inc. with Bird Rock Bio, Inc. surviving as a wholly owned subsidiary of the Company (the “BRB Acquisition”). The purpose of the BRB Acquisition was to acquire BRB's clinical asset, nimacimab, an antibody targeting the CB1 receptor, for development to treat metabolic, inflammatory, and fibrotic conditions.
We were incorporated under the laws of the State of Nevada on March 16, 2011 and are based in San Diego, CA. Since our incorporation, we have devoted substantially all of our efforts to building our product portfolio through the acquisition of clinical assets and licensing agreements, carrying out research and development, building infrastructure and raising capital.
Financial Overview
Revenues
To date, we have not generated any revenue. We do not expect to receive any revenue from any drug candidates that we develop unless and until we obtain regulatory approval for, and commercialize, our drug candidates or generate revenue from collaborative agreements with third parties.
57
Research and Development Expenses
During the year ended December 31, 2023, we incurred $5,819,461 in research and development expenses primarily related to our efforts in conducting the Phase 1 and Phase 2a SBI-100 OE clinical trials. During the year ended December 31, 2022, we incurred $6,011,805 in research and development expense primarily related to our efforts in conducting the Phase 1 SBI-100 clinical trial and the manufacturing of the API required for the Phase 1 and Phase 2a SBI-100 clinical studies. We expect that our ongoing research and development expenses will consist of costs incurred for the development of our drug candidates, including, but not limited to:
| • | license fees; |
|||||||
| • | employee-related expenses, which include salaries, benefits and stock-based compensation; | |||||||
| • | payments to third party contract research organizations and investigative sites; and |
|||||||
| • | payments to third party manufacturing organizations and consultants. |
|||||||
We expect to incur future research and development expenditures to support our nonclinical and clinical studies. Nonclinical activities include, laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess safety and efficacy. Subject to the submission and approval by the FDA of our IND, clinical trials may commence and will involve the administration of the investigational new drug candidate to human subjects.
The process of conducting the necessary clinical research to obtain regulatory approval is costly and time consuming and the successful development of our drug candidates is highly uncertain. Our future research and development expenses will depend on the clinical success of each of our drug candidates, as well as ongoing assessments of the commercial potential of such drug candidates. In addition, we cannot forecast with any degree of certainty which drug candidates may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements. We expect to incur increased research and development expenses in the future as we continue our efforts towards advancing our lead program for nimacimab.
Cost to acquire IPR&D Asset
During the year ended December 31, 2023, we incurred a one-time non-cash charge of $21,215,214 related to the acquisition of our lead clinical asset, nimacimab. This in-process R&D was expensed when purchased in exchange for shares of our common stock, as its only future use was determined to be for drug development.
General and Administrative Expenses
Our general and administrative expenses have fluctuated year-over-year as we have entered into various strategic acquisitions to restructure and re-position our company. Additionally, as a business in the early stages of drug development we are in the process of scaling our operations by hiring additional employees, and building the infrastructure necessary to increase efficiencies. These initiatives have resulted in additional costs related to the implementation of certain systems, insurance, legal and accounting related to operating as a public company. To incentivize our employees and be competitive to retain strong talent we issued additional equity awards in 2023, which have resulted in increased stock-based compensation expense. We expect that our general and administrative expenses will continue to increase in the future in order to support our expected increase in research and development activities, including increased salaries and other related costs, stock-based compensation and consulting fees for executive, finance, accounting and business development functions. We also expect general and administrative expenses to increase as a result of additional costs associated with being a public company, including expenses related to compliance with the rules and regulations of the SEC, additional insurance expenses, investor relations activities and other administration and professional services. Other significant costs are expected to include legal fees relating to patent and corporate matters, facility costs and fees for accounting and other consulting services.
Estimated legal contingency
The estimated legal contingency relates to a wrongful termination suit brought against the former management team that is currently being appealed. As of December 31, 2023, the maximum amount of the liability is known and we have posted an appellate bond that is collateralized by an irrevocable letter of credit equal to, $9,080,202, approximately 150% of the liability recorded on our balance sheet.
Other Expense
Other expense primarily includes interest expense incurred from our short term convertible debt, a loss related to the divestiture of an asset from our 2022 acquisition and an inducement charge from the conversion of debt. In both 2023 and 2022 we also reported wind-down costs from our 2022 acquisition of EHT which we do not expect to incur in future periods. These expenses are offset by interest income earned on our cash balances.
58
Critical Accounting Estimates
Our Management’s Discussion and Analysis of Financial Condition and Results of Operations section discusses our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and the reported amounts of income and expenses during the reporting period. On an on-going basis, management evaluates its estimates and judgments, including those related to accrued expenses, the percentage of completion as it relates to our clinical accruals, financing operations, contingencies, the fair value of assets acquired in the acquisitions, and litigation. Management bases its estimates and judgments on historical experience and on various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. The most significant accounting estimates inherent in the preparation of our consolidated financial statements include estimates as to the appropriate carrying value of certain assets and liabilities which are not readily apparent from other sources. These accounting estimates are described at relevant sections in this discussion and analysis and in the notes to the consolidated financial statements included in this Annual Report on Form 10-K. We believe that the following accounting estimates are the most critical to aid you in fully understanding and evaluating our reported financial results and affect the more significant judgments and estimates that we use in the preparation of our consolidated financial statements.
Accrued Research and Development Expenses
As part of the process of preparing our consolidated financial statements, we are required to estimate our accrued research and development expenses. This process involves reviewing contracts and vendor agreements, communicating with our applicable personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. We make estimates of our accrued and prepaid clinical expenses on a quarterly basis in our consolidated financial statements based on facts and circumstances known to us at that time. Examples of estimated accrued research and development expenses include fees paid to contract research organizations (CROs), investigative sites in connection with clinical studies and to vendors related to product manufacturing and development of clinical supplies.
We base our expenses related to clinical study and trial costs on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical studies on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows and expense recognition. Payments under some of these contracts depend on factors out of our control, such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differ from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. There have been no material changes in estimates for the periods presented.
Stock-Based Compensation Expense
We have stock-based compensation programs, which include restricted stock units (RSUs); stock options and an employee stock purchase plan. We account for stock-based compensation expense, including the expense for grants of stock options and RSUs that may be settled in shares of our common stock, based on the fair values of the equity instruments issued. The fair value is determined on the measurement date, which is generally the date of grant. The fair value of our RSUs is generally measured at the market price of our common stock on the measurement date. Additionally, we use the Monte Carlo Simulation model to evaluate the derived service period and fair value of awards with market conditions, including assumptions of historical volatility, time to the next capital raise and risk-free interest rate commensurate with the vesting term. The fair value for our stock option awards is determined at the grant date using the Black-Scholes valuation model.
Assumptions for the Black-Scholes valuation model used for employee stock awards include:
•Volatility - Stock price volatility is estimated over the expected term based on a blended daily rate of industry peers stock volatility.
•Expected term - The expected term is based on a simplified method which defines the life as the weighted average of the contractual term of the options and the vesting period for each award.
•Risk-free rate - The risk-free interest rate for the expected term of the option is based on the average market rate on U.S. Treasury securities in effect during the period in which the awards were granted.
59
•Dividends - The dividend yield assumption is based on our history and expectation of paying no dividends in the foreseeable future.
We do not believe there is a reasonable likelihood that there will be a material change in the future estimates or assumptions we use to determine stock-based compensation expense. However, if actual results are not consistent with our estimates or assumptions, we may be exposed to changes in stock-based compensation expense that could be material or the stock-based compensation expense reported in our financial statements may not be representative of the actual economic cost of the stock-based compensation.
Recently Issued and Adopted Accounting Pronouncements
See Note 2 to the accompanying consolidated financial statements included in Part IV, Item 15 of this Annual Report on Form 10-K for information on recently issued accounting pronouncements and recently adopted accounting pronouncements. While we expect certain recently adopted accounting pronouncements to impact our estimates in future periods, the impact upon adoption was not significant to our current estimates and operations.
Results of Operations
Comparison of the years ended December 31, 2023 and 2022
Research and Development Expenses
Below is a summary of our research and development expenses during the years ended December 31, 2023 and 2022:
| Year Ended December 31, | ||||||||||||||||||||||||||
| 2023 | 2022 |
$ Change
2023 vs. 2022
|
% Change
2023 vs. 2022
|
|||||||||||||||||||||||
| Research and development expenses | $ | 5,819,461 | $ | 6,011,805 | $ | (192,344) | (3) | % | ||||||||||||||||||
Research and development expenses for the year ended December 31, 2023 decreased by $192,344 when compared to the year ended December 31, 2022. The decrease in research and development expenses was primarily due to a slight delay starting our Phase 2a glaucoma study that we experienced during the second half of 2023. In addition, the decrease in contract manufacturing costs during 2023 was due to the efficient management of sufficient reserves of clinical trial material from our Phase 1 trial to administer the Phase 2a clinical study for glaucoma. The overall decline included a decrease of $422,939 and $210,270 in clinical contract costs and consulting, respectively. Additionally, license fees decreased by $105,356 as the Company achieved a one time milestone payment under our UM 5050 license agreement which was offset by the cancellation of UM 5070. The decreases were offset by increases of $481,411 and $65,296 in research and development salaries and benefits and general business expenses, respectively, due to the expansion of our clinical and R&D team during 2023.
Cost to acquire IPR&D asset
Below is a summary of our cost to acquire the IPR&D asset during the December 31, 2023 and 2022:
| Year Ended December 31, | ||||||||||||||||||||||||||
| 2023 | 2022 | $ Change 2023 vs. 2022 |
% Change 2023 vs. 2022 |
|||||||||||||||||||||||
| Cost to acquire IPR&D asset | $ | 21,215,214 | $ | — | $ | 21,215,214 | 100 | % | ||||||||||||||||||
Cost to acquire the IPR&D asset for the December 31, 2023, increased by $21,215,214 as compared to the year ended December 31, 2022. The increase is due to the cost to acquire nimacimab in the BRB Acquisition.
General and Administrative Expenses
Below is a summary of general and administrative expenses during the years ended December 31, 2023 and 2022:
| Year Ended December 31, | ||||||||||||||||||||||||||
| 2023 | 2022 |
$ Change
2023 vs. 2022
|
% Change
2023 vs. 2022
|
|||||||||||||||||||||||
| General and administrative expenses | $ | 7,852,340 | $ | 6,094,617 | $ | 1,757,723 | 29 | % | ||||||||||||||||||
60
General and administrative expenses for the year ended December 31, 2023 increased by $1,757,723 as compared to the year ended December 31, 2022. The increase in general and administrative expenses was primarily due to an increase in employee wages and board fees of $516,854 related to the addition of three board members and board incentive compensation. Additionally, there were increases in professional and legal fees of $942,336 related primarily to transaction costs associated with the BRB Acquisition, additional regulatory filings, the reverse stock split and ongoing litigation. There was also an increase of $282,447 in general business expenditures due to higher investor relations and travel expenses related to the publicity of our repositioning in 2023 to highlight nimacimab as our lead asset for obesity.
Estimated legal contingency
Below is a summary of the estimated legal contingency during the years ended December 31, 2023 and 2022:
| Year Ended December 31, | ||||||||||||||||||||||||||
| 2023 | 2022 | $ Change 2023 vs. 2022 |
% Change 2023 vs. 2022 |
|||||||||||||||||||||||
| Estimated legal contingency | $ | (151,842) | $ | 6,205,310 | $ | (6,357,152) | N/A | |||||||||||||||||||
Estimated legal contingency for the year ended December 31, 2023 decreased by $6,357,152 as compared to the year ended December 31, 2022. The adjustment to the estimated legal contingency of $151,842 in 2023 was due to the court's determination to decrease the aggregate legal fees owed to the plaintiff in the Cunning Lawsuit.
Other Expense
Below is a summary of other expense during the years ended December 31, 2023 and 2022:
| Year Ended December 31, | ||||||||||||||||||||||||||
| 2023 | 2022 |
$ Change
2023 vs. 2022
|
% Change
2023 vs. 2022
|
|||||||||||||||||||||||
| Change in fair value of derivative liability | $ | (3) | $ | (59,729) | $ | 59,726 | (100) | % | ||||||||||||||||||
| Interest expense | 906,270 | 665,133 | 241,137 | 36 | % | |||||||||||||||||||||
| Interest income | (99,974) | (19,011) | (80,963) | 426 | % | |||||||||||||||||||||
| Finance charge | — | 120,228 | (120,228) | (100) | % | |||||||||||||||||||||
| Loss from asset sale | 307,086 | — | 307,086 | N/A | ||||||||||||||||||||||
| Debt conversion inducement expense | 1,383,285 | — | 1,383,285 | N/A | ||||||||||||||||||||||
| Wind-down costs | 409,347 | 456,508 | (47,161) | (10) | % | |||||||||||||||||||||
| Total other expense, net | $ | 2,906,011 | $ | 1,163,129 | $ | 1,742,882 | 150 | % | ||||||||||||||||||
For the year ended December 31, 2023, we had net other expense of $2,906,011 primarily related to interest expense of $906,270 (including cash and non-cash interest), a non-cash charge of $1,383,285 related to the induced conversion of our Amended Credit Facility, $409,347 in wind down costs associated with the EHT Acquisition and a $307,086 loss from the divestiture of VDL. The increase was offset by interest income of $99,974.
For the year ended December 31, 2022, we had net other expense of $1,163,129 primarily related to interest expense of $665,133 related to the Amended Credit Agreement and wind down costs of $456,508 associated with the EHT Acquisition. In addition, we recognized a finance charge of $120,228 from the repricing of warrants.
Liquidity, Going Concern and Capital Resources
The Company has incurred operating losses and negative cash flows from operations since inception and as of December 31, 2023, had a working capital deficit of $2,250,156 and an accumulated deficit of $104,382,549. As of December 31, 2023, the Company had unrestricted cash in the amount of $1,256,453. For the years ended December 31, 2023 and 2022, the Company incurred losses from operations of $34,735,173 and $18,311,732, respectively. For the years ended December 31, 2023 and 2022, the Company incurred net losses of $37,644,784 and $19,481,602, respectively. The Company expects to continue to incur significant losses and negative cash flows from operations through 2024 and expects to incur significant losses and negative cash flows from operations in the future.
61
Historically, the Company has funded its operations through convertible debt, public equity financings, asset acquisitions and private investments in public equity. On August 18, 2023, the Company entered into the Convertible Note Financing, the August PIPE Financing and BRB Acquisition which provided the Company with the necessary funds to continue operations, post an appeal bond to stay the execution of the judgment in the Cunning Lawsuit and reposition the Company to focus on nimacimab as its lead clinical asset for obesity. Following the August 2023 transactions, the Company executed a 1:250 reverse stock split and increased its authorized shares outstanding. In January 2024 and March 2024, we completed two private placement equity transactions with institutional investors, in which we raised combined net aggregate proceeds of approximately $83,500,000. The capital from the January and March PIPE financings will allow us to fund both of our planned clinical trials for glaucoma and obesity through top line Phase 2 data.
The Company’s consolidated financial statements have been prepared on the basis of the Company continuing as a going concern for the next 12 months. Based on its current operational requirements, the Company believes that its current cash will be sufficient to fund its projected operations for at least 12 months from the date of the issuance of these consolidated financial statements.
Cash Flows
The following is a summary of our cash flows for the periods indicated and has been derived from our consolidated financial statements which are included elsewhere in this Form 10-K:
| Year Ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Net cash and restricted cash provided by (used in): | ||||||||||||||
| Operating activities | $ | (13,952,178) | $ | (12,744,072) | ||||||||||
| Investing activities | 6,596,456 | 5,214,395 | ||||||||||||
| Financing activities | 16,443,270 | (208,794) | ||||||||||||
Net increase (decrease) in cash and restricted cash |
$ | 9,087,548 | $ | (7,738,471) | ||||||||||
Cash Flows from Operating Activities
The primary use of cash for our operating activities during these periods was to fund research and development activities for our clinical product candidates, nimacimab and SBI-100 OE, along with general and administrative activities. Our cash used in operating activities also reflected changes in our working capital, net of adjustments for non-cash charges, such as , stock-based compensation expense, non-cash interest expense related to the amortization of debt discounts on our convertible debt instruments, a charge to induce the conversion of the Amended Credit Agreement during February 2023 and the expense related to the acquisition of our lead asset for obesity, nimacimab.
Cash used in operating activities of $13,952,178 during the year ended December 31, 2023, reflected a net loss of $37,644,784, the loss was adjusted by aggregate non-cash charges of $24,161,912 and included a $469,306 decrease in our operating assets and liabilities. Non-cash charges included $124,251 of depreciation and amortization, $987,510 for stock-based compensation expense, $329,890 in non-cash interest expense from the amortization of the debt discount on our convertible debt, a gain of $151,843 from the courts decision to reduce the legal fees due to the plaintiff in the Cunning Lawsuit, $307,086 for a non-cash loss on the divestiture of VDL, a debt conversion inducement charge of $1,383,285 related to the conversion of the multi-draw credit agreement and in-process research and development expenses of $21,215,214 related to the acquisition of our lead asset, nimacimab. The net change in our operating assets and liabilities included a $306,442 increase in our prepaid expense and other current assets, a decrease in accounts payable of $701,285, and a $74,463 decrease in our accrued expense and other current liabilities.
Cash used in operating activities of $12,744,072 during the year ended December 31, 2022, reflected a net loss of $19,481,602, partially offset by aggregate non-cash charges of $7,499,434 and included a $761,904 net change in our operating assets and liabilities. Non-cash charges included $629,032 for stock-based compensation expense, $489,595 non-cash interest expense from the amortization of the debt discount on the Amended Credit Agreement, a $59,729 gain from the decrease in fair value of our warrant liability, depreciation and amortization of $114,998, a finance charge of $120,228 due to Sciences warrant repricing, and a loss of $6,205,310 due to the estimated legal contingency associated with the Cunning Lawsuit. The net change in our operating assets and liabilities included a $109,943 increase in our prepaid expense and other current assets, an increase in accounts payable of $799,740, and a $1,671,587 decrease in our accrued expense and other current liabilities.
62
Cash Flows from Investing Activities
Cash provided from investing activities of $6,596,456 during the year ended December 31, 2023 consisted of our capital expenditures in relation to the purchase of property plant and equipment of $12,550, cash divested net of proceeds received from the sale of VDL of $5,532,266 and cash proceeds received from the BRB Acquisition of $1,076,740. During the year ended December 31, 2022, the Company purchased $28,060 of machinery and office equipment, cash divested net of proceeds received from the sale of an asset of $66,458 and cash proceeds received from the EHT Acquisition of $5,308,913.
Cash Flows from Financing Activities
During the year ended December 31, 2023, cash provided by financing activities included $11,734,947 in net proceeds received from the August 2023 PIPE Financing, $4,973,684 in net proceeds from the issuance of a convertible note, offset by $259,335 in repayments on our insurance premium financing.
During the year ended December 31, 2022 cash used in financing activities included $1,967 in proceeds received in connection with pre-funded warrants and $680,901 in proceeds from the EHT bridge financing, offset by $275,537 in repayments on our insurance premium financing, and $616,125 in prepayments on the Amended Credit Agreement.
Off-Balance Sheet Arrangements
There are no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable.
Item 8. Financial Statements and Supplementary Data.
Our consolidated financial statements and the report of our independent registered public accounting firm are included in this report on pages F-1 through F-38
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
Not applicable.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We maintain controls and procedures that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive and principal financial officers, as appropriate, to allow timely decisions regarding required disclosures. Based upon their evaluation of those controls and procedures performed as of the end of the period covered by this report, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting as defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, a company’s principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP and includes those policies and procedures that:
•pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of the Company;
•provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with GAAP, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the Company; and
•provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
63
Because of its inherent limitations, our internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management, with the supervision and participation of our Chief Executive Officer and Chief Financial Officer, assessed the effectiveness of our internal control over financial reporting as of December 31, 2023, based on criteria for effective internal control over financial reporting set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework - 2013 (COSO 2013 Framework).
Based on their assessment, our management concluded that, as of December 31, 2023, our internal control over financial reporting was effective.
As we are a smaller reporting company, our independent registered public accounting firm is not required to attest to the effectiveness of our internal control over financial reporting.
Changes in internal control over financial reporting
There was no change in our internal control over financial reporting during the fourth quarter ended December 31, 2023 that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
64
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The following table sets forth certain information as of the date of this Annual Report, with respect to our directors, executive officers and significant employees.
| Name | Age | Position | ||||||||||||
| Punit Dhillon | 43 |
Chief Executive Officer, Chairman, Director | ||||||||||||
| Kaitlyn Arsenault | 37 |
Chief Financial Officer | ||||||||||||
Tuan Tu Diep |
43 |
Chief Development Officer |
||||||||||||
| Margaret Dalesandro | 77 |
Director | ||||||||||||
| Deborah Charych | 59 |
Director | ||||||||||||
| Praveen Tyle | 64 |
Director | ||||||||||||
| Keith Ward | 54 |
Director | ||||||||||||
Andy Schwab |
53 |
Director |
||||||||||||
Paul Grayson |
59 |
Director |
||||||||||||
Annalisa Jenkins |
58 |
Director |
||||||||||||
Biographies of Directors, Executive Officers and Significant Employees
Punit Dhillon. Punit Dhillon currently serves as the Chair of the Board of Directors and as the Company’s President and Chief Executive Officer. Mr. Dhillon was appointed as a member of the Board of Directors in January 2018. In December 2019, Mr. Dhillon was appointed as the Chairman of the Board of Directors. In August 2020, Mr. Dhillon was appointed as the Company's Chief Executive Officer. Mr. Dhillon is currently a board member and audit committee chair of Arch Therapeutics Inc., a US-based biotechnology company (OTCQB: ARTH). Mr. Dhillon was the co-founder and former President & CEO of OncoSec Medical, Inc. (NASDAQ: ONCS), a leading biopharmaceutical company developing cancer immunotherapies for the treatment of solid tumors, where he served as an executive until March 2018 and as a director until February 2020. He led OncoSec through over $250 million in capital raised, NASDAQ listing and launched the registration study, KEYNOTE695, of their proprietary immunotherapy product for melanoma in combination with Keytruda, based on a drug collaboration with Merck. Prior to that, from September 2003 to March 2011, Mr. Dhillon served as Vice President of Finance and Operations at Inovio Pharmaceuticals, Inc. (NASDAQ: INO), a DNA vaccine development company. From February 2017 to August 2020, Mr. Dhillon was a director of Emerald Health Sciences a private company that made strategic equity investments related to endocannabinoid based science and clinical products. Collectively, Mr. Dhillon has led and assisted in raising over $500 million through financings and mergers and acquisitions deals, as well as several licensing and development transactions with large pharmaceutical companies including Merck & Co., Inc. (NYSE: MRK), Bristol Myers Squibb Co (NYSE: BMY), and Pfizer Inc. (NYSE: PFE). Mr. Dhillon also co-founded and is the director of YELL Canada, a registered Canadian charity that partners with schools to support entrepreneurial learning. Mr. Dhillon received his Bachelor of Arts Honors degree in Political Science with a minor in Business Administration from Simon Fraser University. Mr. Dhillon's experience in the biotechnology and pharmaceutical industry and his experience with publicly traded companies give him the qualifications necessary to serve as an officer and director of the Company.
Kaitlyn Arsenault, CPA. Kaitlyn Arsenault was appointed as the Company’s Chief Financial Officer in October 2021. From 2014 to 2021, Ms. Arsenault served as the President of KA Consulting, Inc., a registered public accounting firm in San Francisco, CA, providing independent technical accounting consulting services for emerging public and private companies in the pharmaceutical, life sciences, technology, and FinTech industries. From September 2016 to October 2021, she served as the Company’s Head of Financial Reporting and Technical Accounting. Ms. Arsenault's experience includes addressing complex technical accounting issues related to equity financings, derivatives, debt instruments, stock-based compensation, revenue recognition, and mergers and acquisitions, among other subjects. Prior to becoming an independent financial consultant, Ms. Arsenault spent seven years in public accounting as an assurance manager in the SEC practice of Friedman LLP (now Marcum LLP), gaining public and private audit engagement experience across multiple industries. Ms. Arsenault received her Bachelor of Science degree in Accounting from Ramapo College of New Jersey and is a Certified Public Accountant in California (active) and New Jersey (inactive). Ms. Arsenault's prior track record with the Company, extensive experience with pharmaceutical, life science, and technology companies, and vast exposure to different accounting and financial issues in the public markets give her the qualifications and skills necessary to serve as an officer of the Company.
65
Tuan Tu Diep. Tuan Tu Diep was appointed as the Company’s Chief Development Officer in January 2022, after serving as the Company’s Senior Vice President of Development from October 2020 to January 2022. From March 2020 to October 2020, Mr. Diep served as the Director of Business Process of Element Bioscience, a next-generation sequencing company focused on the the development of revolutionary and proprietary technology to deliver high-quality sequencing on their benchtop instrument, AVITI. In addition, he served as the President of Emerald Health Bioceuticals from October 2019 through January 2020. From July 2018 to October 2019, Mr. Diep served as the Vice President of Strategic Operations of Emerald Health Sciences USA, a private life science company that made strategic equity investments related to endocannabinoid based science and clinical products. Mr. Diep was a founding employee of OncoSec Medical Inc. (NASDAQ:ONCS) a leading biopharmaceutical company developing cancer immunotherapies for the treatment of solid tumors, where he served in multiple roles of increasing responsibility from 2011 to 2018. Here he led the initiation of OncoSec's first clinical trials in melanoma nd merkel cell carcinoma. As OncoSec's business development lead he was instrumental in establishing a partnership and clinical collaboration with Merck & Co. Inc. (NYSE: MRK) for the evaluation of Keytruda with OncoSec's lead drug, tavokinogene telsaplasmid and launched the pivotal trial, KEYNOTE-695. Mr. Diep is an experienced executive that has taken multiple drugs from early preclinical development to the clinic. He has demonstrated a proficiency in managing the initiation and execution of multiple clinical trials; including the manufacture, release, and distribution of drugs and devices for clinical use; managing the completion of financial audits for both private and public companies; overseeing due diligence activities related to partnering and licensing initiatives; and playing significant roles in raising funds in public markets. Mr. Diep received his Bachelor degree in Human Kinetics from the University of British Columbia in 2004 and his Masters of Science from the University of Toronto in 2006. Mr. Diep’s vast experience with life science companies gives him the qualifications and skills necessary to serve as an officer of the Company.
Dr. Margaret Dalesandro. Dr. Margaret Dalesandro is currently a member of the Board and has served as a member since August 2020. From 2019 to 2021, Dr. Dalesandro served on the board of OncoSec Medical Incorporated (NASDAQ: ONCS), a late-stage biotechnology company focused on designing, developing, and commercializing innovative therapies and proprietary medical approaches to stimulate and guide an anti-tumor immune response for the treatment of cancer. In addition, she served as Chair of the OncoSec Medical Incorporated Board from early 2020 through 2021. Since 2021, Dr Dalesandro has served on the board and chaired the Nominating and Corporate Governance Committee of Seelos Therapeutics (NASDAQ:SEEL), a company focusing on the development of treatments for central nervous system diseases including ALS. Since 2023, Dr. Dalesandro has served on the Board of Ambrx Biopharma Inc (NASDAQ: AMAM), a company expert in the development of antibody drug conjugates (ADCs), immune-oncology and bispecific candidates for the treatment of cancers including prostate and breast cancer. In January 2024, Ambrx announced an agreement of sale to Johnson & Johnson for $2 billion. Since 2012, Dr. Dalesandro has been the President of Brecon Pharma Consulting LLC., a full-service pharma/biotech consultancy focusing on technical due diligence and creating strategic development plans identifying and obtaining critical information early in pharma/biotech product development. Dr. Dalesandro has over thirty-five years of experience leading strategic product development in the pharmaceutical, biotechnology, and diagnostics industries. From 2009 to 2012, she served as the Business Director of Integrative Pharmacology in the Life Sciences (Corning Integrative Pharmacology - CIP) division at Corning Incorporated (NYSE:GLW), leading all aspects of the CIP business including commercial, technical, P&L, competitive assessment, strategy, and talent management; from 2002 to 2009, as Vice President of Project, Portfolio, and Alliance Management at ImClone Systems Incorporated, which was a biopharmaceutical company dedicated to developing biologic medicines in the area of oncology and purchased by Eli Lilly (NYSE:LLY) for $6 billion. During her time at ImClone Systems, she contributed significantly to the development and approval of breakthrough oncology drugs including: Erbitux, Cyramza and Lartruvo; from 2000 to 2002, as Executive Director of Project and Portfolio Management at GlaxoSmithKline, a global pharmaceutical company producing treatments for respiratory illnesses, HIV, immuno-inflammation, and oncology (among others) (NYSE: GSK); and from 1998 to 2000, as Senior Consultant at Cambridge Pharma Consultancy, Europe's largest pharmaceutical R&D strategy consulting firm. During her tenure from 1989 to 1998 at Centocor Incorporated, a biotechnology company forming a part of the Johnson & Johnson group of companies and specializing in the production of antibody treatments for infectious, cardiovascular, and autoimmune diseases and cancer, Dr. Dalesandro played a key role in the development of Remicade, the first anti-TNF alpha antibody developed for autoimmune disease and ReoPro for the prevention of myocardial ischemia. She also holds the patents on a diagnostic test for acute coronary syndrome based on the detection of platelet surface integrins. Dr. Dalesandro received her Ph.D. in Biochemistry from Bryn Mawr College and completed an NIH Post-Doctoral Fellowship in Molecular Immunology at Wake Forest University School of Medicine and the University of Pennsylvania.. Dr. Dalesandro’s extensive experience with life science and technology companies gives her the qualifications and skills necessary to serve as a director of the Company.
66
Dr. Deborah Charych. Dr. Deborah Charych is currently a member of the Board and has served as a member since February 2023. From October 2018 to September 2022, Dr. Charych has served as the Co-Founder, Chief Technology Officer, and Advisor of RayzeBio, Inc, an oncology company focused on the targeted delivery of radionuclides. Dr. Charych conceived and led the scientific and operational R&D strategy for RayzeBio, recently acquired by BMS for $4.1 billion, leading a successful Series A financing and launch in August 2020, as well as subsequent Series B, C, and D rounds. Prior to launching RayzeBio, Dr. Charych held a number of scientific leadership positions in biotech focused on translational drug development. From 2017 to 2019, she founded Third Rock Ventures, creating new biotech companies based on strong science, co-founding Maze Therapeutics, which focuses on harnessing the power of human genetics, functional genomics, and data science to advance our understanding of how to more effectively treat patients with severe rare and common diseases. From 2010 to 2018, Dr. Charych served as Executive Director of Preclinical and Translational Research at Nektar Therapeutics, conceiving of and leading the pre-clinical and early clinical development of an immuno-oncology pipeline with NKTR-214 and NKTR-358, next-generation IL-2 receptor agonists, which are currently in Phase 3 oncology and Phase 2 autoimmune clinical trials. At FivePrime Therapeutics from 2007 to 2010, Dr. Charych was the Director of Biologics Process Development/CMC/Protein Chemistry, leading a team that contributed to the clinical development of novel biologics for pan-FGF and CSF1 antagonist antibodies for oncology and immunology diseases. From 1998 to 2006, while at Chiron Corporation, she initiated and led a large proteomics effort to guide oncology target discovery, including the discovery of peptide-mimetic binders ('peptoids'). During her time at Lawrence Berkeley National Laboratory from 1993 to 1998, she assumed an academic leadership role as a tenured Principal Investigator, focusing on new biomaterials. Dr. Charych earned a PhD in Physical Chemistry from the University of California in Berkeley, CA and a B.S. in Chemistry from Carnegie-Mellon University in Pittsburgh, PA. Dr. Charych’s education and significant experience with a wide variety of life science companies give her the qualifications and skills necessary to serve as a director of the Company.
Dr. Praveen Tyle. Dr Tyle is currently the Founder of Potens Pharmaceuticals, which is focussed on helping companies develop drug development programs with speed to market. Dr. Praveen Tyle is currently a member of the Board and has served as a member since July 2021. Since 2006, Dr. Tyle has served as a member of the board at Kiora Pharmaceuticals, a pharmaceutical company that develops therapies for the treatment of eye diseases (NASDAQ: KPRX) and currently serves as its Chairman and since 2003, he has served as a member of the board at Orient Europharma Co., Ltd., a pharmaceutical company operating primarily in Asia and producing a wide range of prescription drugs and nutrition products. Since 2021, Dr. Tyle has served as President, Chief Executive Officer, and Director of Invectys, Inc., a clinical-stage biopharmaceutical company founded from the world-renowned Pasteur Institute and focused on the development of innovative immunotherapy approaches to treat cancers. From 2016 to 2021, he was Executive Vice President of Research and Development at Lexicon Pharmaceuticals, Inc., a pharmaceutical company whose genetic approach to drug development is based on Nobel Prize-winning technology (NASDAQ: LXRX). From 2013 to 2016, he served as President, Chief Executive Officer, and Director of Osmotica Holdings (Cyprus & Osmotica Pharmaceutical), a company focusing on central nervous system drug development. From 2011 to 2012, Dr. Tyle was the Executive Vice President and Chief Scientific Officer of United States Pharmacopeia, an independent scientific nonprofit organization focused on building trust in the supply of safe, quality medicines. From 2008 to 2010, Dr. Tyle served as Senior Vice President and Global Head of Business Development and Licensing and Global Head of Research and Development at Novartis OTC, a pharmaceutical company that produces both patented and generic product on a global scale (NYSE: NVS). Earlier in his career, from 2004 to 2008, he was Corporate Senior Vice President and Chief Scientific Officer at Bausch + Lomb Corporation, a company specializing in eye care and whose products and innovations range from pharmaceuticals, lenses, and diagnostic and surgical tools (NYSE: BLCO / TSX: BLCO). Since 2005, Dr. Tyle has served as an Adjunct Associate Professor of Ophthalmology at the University of Rochester Eye Institute Medical Center, among other current and past academic roles. He has coauthored over 100 peer-reviewed academic papers and presentations and is named on multiple patents, including those related to ophthalmic innovations, drug delivery, and glaucoma. Dr. Tyle earned his B.Pharm. from Banaras Hindu University in India and received his PhD in Pharmaceutics & Pharmaceutical Chemistry from Ohio State University. Dr. Tyle's significant contributions in the field of ophthalmology and extensive experience with life science companies give him the qualifications and skills necessary to serve as a director of the Company.
67
Dr. Keith Ward. Dr. Keith Ward is currently a member of the Board and has served as a member since December 2021. Dr. Ward is a life sciences executive with over twenty-five years of experience in the biotech and pharmaceutical industry. In 2022, Dr. Ward co-founded Kuria Therapeutics, a private pharmaceutical company developing novel ophthalmic and dermal therapeutics, where he currently serves as President and Chief Executive Officer. Since 2019, Dr. Ward has also served as President and Chief Executive Officer of InterveXion Therapeutics, a private clinical-stage biotech company developing immunotherapies for substance use disorders. Prior to joining InterveXion, Dr. Ward served as Executive Vice President and Chief Development Officer for Reata Pharmaceuticals, where he led research and development, clinical operations, regulatory affairs, manufacturing, and project management. Before that, Dr. Ward developed ophthalmic pharmaceuticals and medical devices as Global Vice President of Pharmaceutical R&D for Bausch + Lomb. Dr. Ward has also held positions of increasing responsibility within GlaxoSmithKline and SmithKline Beecham Pharmaceuticals. Dr. Ward earned a B.S. in Toxicology with a minor in Chemistry from Northeast Louisiana University and a Ph.D. in Toxicology from the University of North Carolina at Chapel Hill. Dr. Ward’s significant experience in biotech and pharmaceutical companies give him the qualifications and skills necessary to serve as a director of the Company.
Andy Schwab. Andrew Schwab is a Founding Partner and Managing Member of 5AM Venture Management, LLC, a venture capital firm focused on life science investments founded in 2002. Mr. Schwab was previously a Principal at Bay City Capital, a life sciences investment firm, where he was involved with companies such as Cubist Pharmaceuticals, Inc., PTC Therapeutics, Inc., Symyx Technologies, Inc. and Syrrx, Inc. Previously, Mr. Schwab was Vice President of Business Development at Digital Gene Technologies, Inc., and a Vice President in the life science investment banking group of Montgomery Securities. At 5AM, Mr. Schwab has led the firm’s investments in and currently serves on the Board of Directors of Skye Bioscience, Inc. (formerly Bird Rock Bio, Inc.), Camp4 Therapeutics Corporation, Escient Pharmaceuticals, Inc., Fellow Health, Inc., Novome Biotechnologies, Inc., Radionetics Oncology, Inc., Rarecyte, Inc., Scientist.com, and TMRW Life Sciences, Inc. Mr. Schwab previously served on the Board of Directors of various companies, including BlueLight Therapeutics, Inc, Cleave Therapeutics, Inc., DVS Sciences, Inc. (which was acquired by Fluidigm Corporation), Enliven Therapeutics, Inc., Flexion Therapeutics, Inc., Ikaria, Inc. (which was acquired by Mallinckrodt plc and spun out Bellerophon Therapeutics, Inc.), Ilypsa, Inc. (which was acquired by Amgen, Inc.), Miikana Therapeutics, Inc. (which was acquired by EntreMed, Inc.), Panomics Inc. (which was acquired by Affymetrix, Inc.), Pear Therapeutics, Inc., Precision NanoSystems, Inc. (which was acquired by Danaher Corporation), Purigen Biosystems, Inc., Synosia Therapeutics Holding AG (which was acquired by Biotie Therapies Corp.), Viveve Medical, Inc., and 5:01 Acquisition Corp. Mr. Schwab also currently serves on the boards of trustees of the California Academy of Sciences and Davidson College. He holds a B.S. degree with Honors in Genetics & Ethics from Davidson College. Mr. Schwab’s extensive experience in the biotechnology industry give him the qualifications and skills necessary to serve as a director of the Company.
Paul Grayson. Paul Grayson has served as President and Chief Executive Officer of Radionetics Oncology, a clinical stage biotechnology company focused on novel radiopharmaceutical products, since November 2023. From July 2020 to November 2023 President and Chief Executive Officer of Tentarix Biotherapeutics Inc., a biotechnology company, and as President and Chief Executive Officer of Bird Rock Bio, Inc., a clinical stage biopharmaceutical company, from June 2011 until its acquisition by the Company. From November 2019 to July 2020, Mr. Grayson also served as a partner at Versant Ventures, a venture capital firm. Mr. Grayson currently serves on the board of directors of Radionetics Oncology. He received a Bachelor of Arts in Biochemistry and Computer Science from the University of California, Los Angeles and a Master of Business Administration from the University of California, Irvine. Mr. Grayson’s extensive experience in the biotechnology industry give him the qualifications and skills necessary to serve as a director of the Company.
Annalisa Jenkins. Dr. Annalisa Jenkins is currently a member of the Board and has served as a member since March 2024. From November 2017 until April 2019, Dr. Jenkins served as the President and Chief Executive Officer of PlaqueTec Ltd., a biotechnology company focusing on coronary artery disease treatment and prevention. Previously, Dr. Jenkins served as the President and Chief Executive Officer and a member of the board of directors of Dimension Therapeutics, Inc., a biotechnology company focused on rare and metabolic diseases associated with the liver, from September 2014 until its sale to Ultragenyx Pharmaceutical Inc. in November 2017. Dr. Jenkins also serves on the board of directors of many public companies, including Avrobio, Inc. (Nasdaq: AVRO), Affimed N.V. (Nasdaq: AFMD), Compass Pathways (Nasdaq: CMPS), Mereo Biopharma Group plc (Nasdaq: MREO), and a number of privately held biotechnology and life science companies, and serves as a trustee to a number of non-profit organizations. Dr. Jenkins previously served on the board of numerous biotechnology and life science companies, including AgeX Therapeutics, Inc. (NYSE American: AGE), Silence Therapeutics, Ardelyx, Inc., OncoSec Medical Incorporated, and Sensyne Health plc., and she served as a committee member of the science board to the FDA, which advised leadership on complex scientific and technical issues. Dr. Jenkins also previously held leadership roles at Merck Serono Pharmaceuticals as Head of Global Research and Development, and at Bristol-Myers Squibb as Senior Vice President and Head of Global Medical Affairs. Dr. Jenkins graduated with a degree in medicine from St. Bartholomew’s Hospital in the University of London and subsequently trained in cardiovascular medicine in the UK National Health Service. Earlier in her career, Dr. Jenkins served as a medical officer in the British Royal Navy. Dr. Jenkins significant industry experience and training give her the qualifications and skills necessary to serve as a director of the Company.
68
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors, executive officers, and any persons who own more than 10% of a registered class of our equity securities, to file reports of ownership and changes in ownership with the SEC. SEC regulation requires executive officers, directors and greater than 10% stockholders to furnish us with copies of all Section 16(a) forms they file. Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that during the year ended December 31, 2023, our executive officers, directors, and greater than 10% stockholders complied with all applicable filing requirements on a timely basis.
Family Relationships
There are no family relationships among our directors or executive officers.
Term of Office of Directors
Our directors serve until the next annual meeting of stockholders or until their successor has been duly elected and qualified, or until their earlier death, resignation or removal.
Directors and Officers Involvement in Certain Legal Proceedings
During the past ten years, our current directors and executive officers have not been involved in any of the legal proceedings set forth in Item 401(f) of Regulation S-K promulgated by the SEC.
Board and Committee Meetings
During 2023, our Board met eight times (including telephonic meetings) and took action by written consent twenty-two times. Each director attended at least 75% of the meetings held by the Board and by each committee on which she or he served while she or he was a director, either in person or by teleconference, during the year. During 2023, our Board met by special committee eleven times (including telephonic meetings) and took action by written consent two times as a result of the special committee meetings.
Director Attendance at Annual Meetings
Although we do not have a formal policy regarding attendance by members of our Board at each annual meeting of stockholders, we encourage all of our directors to attend. All our directors - other than Dr. Charych, Andy Schwab, Paul Grayson and Dr. Annalisa Jenkins who were elected as directors in 2023 and 2024 - attended our most recent meeting of stockholders in 2022.
Audit Committee and Financial Expert
Our Audit Committee is composed of Dr. Keith Ward, Dr. Margaret Dalesandro and Dr. Praveen Tyle. Dr. Ward is the chairperson of our Audit Committee. Dr. Keith Ward, Dr. Margaret Dalesandro and Dr. Praveen Tyle meet the requirements for independence for audit committee members under the SEC rules and regulations. Each member of our Audit Committee is financially literate. In addition, our board has determined that Dr. Ward is an “audit committee financial expert” as defined in applicable SEC rules. This designation does not impose any duties, obligations, or liabilities that are greater than are generally imposed on members of our Audit Committee and our Board. Our Audit Committee is responsible for, among other things: our accounting and financial reporting processes, including our financial statement audits and the integrity of our financial statements; our compliance with legal and regulatory requirements; reviewing and approving related party transactions; selecting and hiring our registered independent public accounting firm; the qualifications, independence and performance of our independent registered public accountants; and the preparation of the audit committee report to be included in our annual proxy statement.
During the fiscal year ended December 31, 2023, the Audit Committee met five times.
Compensation Committee
Our Compensation Committee is composed of Dr. Praveen Tyle, Dr. Margaret Dalesandro and Dr. Annalisa Jenkins. Dr. Praveen Tyle is the chairperson of our Compensation Committee. The composition of our Composition Committee meets the requirements for independence under the SEC rules and regulations. Our Compensation Committee is responsible for, among other things: evaluating, recommending, approving and reviewing executive officer compensation arrangements, plans, policies and programs; administering our cash-based and equity-based compensation plans; and making recommendations to the Board regarding any other Board responsibilities relating to executive compensation.
During the fiscal year ended December 31, 2023, the Compensation Committee met five times.
69
Nomination and Corporate Governance Committee
Our Nominating and Corporate Governance Committee is composed of Dr. Margaret Dalesandro, Dr. Praveen Tyle, Dr. Keith Ward, Dr. Annalisa Jenkins and Dr. Deborah Charych. Dr. Charych is the chairperson of our Nominating and Corporate Governance Committee. The composition of our Nominating and Corporate Governance Committee meets the requirements for independence under the SEC rules and regulations. Our Nominating and Corporate Governance Committee is responsible for, among other things: identifying, considering and recommending candidates for membership on our Board; overseeing the process of evaluating the performance of our Board; and advising our Board on other corporate governance matters.
During the fiscal year ended December 31, 2023, the Nominating and Corporate Governance Committee met five times.
Nominations to the Board of Directors
We do not have any defined policy or procedural requirements for shareholders to submit recommendations or nominations for directors. Our Board believes that, given the stage of our development, a specific nominating policy would be premature and of little assistance until our business operations develop to a more advanced level. We do not currently have any specific or minimum criteria for the election of nominees to the Board. The Board, with the help of its nomination and corporate governance committee, will assess all candidates and make recommendations for election or appointment.
Stockholder Communications
We do not have a formal policy regarding stockholder communications with our Board. A shareholder who wishes to communicate with our Board may do so by directing a written request addressed to our Chief Executive Officer, at the address appearing on the first page of this filing.
Code of Ethics
The Board has established a formal code of business conduct and ethics that applies to our officers, directors and employees. Any amendment or waiver disclosed on our website will remain available on our website for at least 12 months after the initial disclosure. Any waiver of the code of business conduct and ethics for our executive officers or directors must be approved by the Board, and any such waiver shall be promptly disclosed to the stockholders.
Insider Trading Policy
We maintain a Policy on Insider Trading and Insider Information that prohibits our officers, directors and employees from purchasing or selling any type of security while in possession of material, non-public information relating to the security, whether the issuer of such security is the Company or any other company. Additionally, no officer, director or employee shall purchase or sell any security of the Company during the period beginning on the 15th calendar day of the last month of each fiscal quarter of the Company and ending upon completion of the second full trading day after the public release of earnings data for such fiscal quarter or during any other trading suspension period declared by the Company. It prohibits officers, directors, or employees from pledging our stock as collateral to secure loans and from engaging in hedging transactions, including zero-cost collars and forward sale contracts. It further prohibits margin purchases of our stock, short sales of our stock, and any transactions in puts, calls or other derivative securities involving our stock.
Availability of Corporate Governance Materials
Stockholders may view our corporate governance materials, including the charters of the Audit Committee, Compensation Committee, and Nominating and Corporate Governance Committee and our Code of Business Conduct and Ethics, on our website at www.skyebioscience.com under “Governance” on the “Investors” page, and these documents are available in print to any stockholder who sends a written request to such effect to Skye Bioscience, Inc., 11250 El Camino Real, Suite 100, San Diego, CA 92130, Attention: Corporate Secretary. Information on or accessible from our website is not and should not be considered a part of this Annual Report on Form 10-K.
70
Item 11. Executive Compensation.
The Company effected a reverse stock split at a ratio of one-for-two hundred and fifty (1-for-250) effective September 8, 2023 (the “Reverse Stock Split”). All share amounts and exercise prices included herein have been adjusted to reflect the Reverse Stock Split.
Summary Compensation Table
The following table sets forth information concerning the compensation earned for services rendered to us for the fiscal years ended December 31, 2023 and 2022 of our named executive officers as determined in accordance with SEC rules.
| SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||||||||||||||||||||
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) (2) |
Stock Awards ($) (1) |
Option Awards ($) (1) |
Non-Equity Incentive Plan Compensation ($) |
Total ($) |
|||||||||||||||||||||||||||||||||||||
| Punit Dhillon | 2023 | 450,000 | — | 994,364 | 92,974 | 270,000 | 1,807,338 | |||||||||||||||||||||||||||||||||||||
| CEO | 2022 | 432,577 | 74,000 | — | — | 161,904 | 668,481 | |||||||||||||||||||||||||||||||||||||
| Kaitlyn Arsenault | 2023 | 340,000 | — | 656,234 | 138,056 | 136,000 | 1,270,290 | |||||||||||||||||||||||||||||||||||||
| Chief Financial Officer | 2022 | 325,856 | 55,500 | — | — | 87,731 | 469,087 | |||||||||||||||||||||||||||||||||||||
___________
(1)Amounts reflect the full grant date fair value of RSUs, computed in accordance with ASC Topic 718 - Stock based compensation, rather than the amounts paid to or realized by the named executive officers. The valuation assumptions used in the valuation of options and RSUs may be found in Note 2 to our financial statements included in this annual report on Form 10-K for the year ended December 31, 2023. The amount reported is also the amount that would be reported assuming the highest level of performance conditions are achieved. The restricted stock units vest upon achievement of the following performance milestones, subject to continued services to the Company through the applicable vesting date: an incremental 25% of the restricted stock units vest upon the Company achieving a market capitalization of $125 million, $250 million, $400 million, respectively, and an additional 25% vests if the Company achieves a value of $500 million or greater at an exit event. If the Company achieves an exit value greater than $500 million at an earlier date, subject to the executive’s continued services with the Company through such exit event, all of the restricted stock units will vest. No restricted stock units will vest until the compensation committee of the Company determines that shares can be sold into the market to cover withholding tax obligations associated with the vesting of the restricted stock units. As of December 31, 2023, no market capitalization milestone was achieved and no restricted stock units were vested. Amounts reflect the full grant date fair value of stock options, computed in accordance with ASC Topic 718 - Stock based compensation, rather than the amounts paid to or realized by the named executive officers. The value of stock option awards was estimated using the Black-Scholes option pricing model. The valuation assumptions used in the valuation of options and restricted stock units may be found in Note 2 to our financial statements included in this annual report on Form 10-K for the year ended December 31, 2023.
(2)Amounts reflect the cash bonuses earned by our named executive officers for performance of services in 2023 and 2022. Bonuses were based upon achievement of corporate performance goals as determined by the Board.
Narrative Disclosure to Summary Compensation Table
Our compensation committee has historically determined the compensation of our named executive officers. Our compensation committee typically reviews and discusses management’s proposed compensation with the Chief Executive Officer for all executives other than the Chief Executive Officer. Based on those discussions and its discretion, the compensation committee then approves the compensation of each executive officer after discussions without members of management present.
71
Base Salary
Base salaries for our named executive officers are initially established through arm’s-length negotiations at the time of the executive officer’s hiring, taking into account such executive officer’s qualifications, experience, the scope of his or her responsibilities and competitive market compensation paid by other companies for similar positions within the industry and geography. Annual base salaries are intended to provide a fixed component of compensation to our named executive officers, reflecting their skill sets, experience, roles and responsibilities. Base salaries are reviewed, determined, and approved periodically, typically in connection with our annual performance review process, and adjusted from time to time to realign salaries with market levels after taking into account individual responsibilities, performance and experience. The annualized 2023 base salaries for our named executive officers were as follows: (i) $450,000 for Mr. Dhillon and (ii) $340,000 for Ms. Arsenault.
Annual Performance-Based Compensation
In addition to base salaries, our named executive officers are eligible to receive annual performance-based bonuses, which are designed to provide appropriate incentives to our executive officers to achieve annual performance goals and to reward them for achievement towards these goals. Performance based milestones are approved by the board at the beginning of the year and executive officers are assessed against these performance-based milestones subsequent to year end. With respect to 2023, our compensation committee awarded a bonus of $161,904 to Mr. Dhillon, a bonus of $87,731 to Ms. Arsenault. Please see “Employment and Severance Agreements —Employment Agreements” below for additional information.
Equity Incentives
We believe that our ability to grant equity-based awards is a valuable and necessary compensation tool that aligns the long-term financial interests of our employees, consultants and directors with the financial interests of our shareholders. Our compensation committee periodically reviews the equity incentive compensation of our executive officers, including our named executive officers, and from time to time may grant equity incentive awards to them.
In August 2023, we completed the acquisition of Bird Rock Bio, Inc. and closed a concurrent private placement financing and a convertible note financing. Following the completion of the transaction, our compensation committee, and its advisors undertook a review of the compensation of our executive officers, directors and employees.
Following such review, on August 25, 2023, we granted each of Mr. Dhillon and Ms. Arsenault (i) an option to purchase 9,013 and 13,383 shares of our common stock, respectively, at an exercise price per share of $3.50 and (ii) a contingent option award to, purchase 21,586 and 32,053 shares of our common stock, respectively, at an exercise price per share of $3.50, subject to the approval of an amendment to our Amended and Restated 2014 Omnibus Incentive Plan (the “A&R 2014 Incentive Plan”) to increase the number of shares authorized for issuance under the A&R 2014 Incentive Plan (the “Plan Amendment”). On September 29, 2023 holders of a majority of the voting power of the outstanding capital stock of the Company (the “Majority Stockholders”) and the Board approved the Plan Amendment. These options vest in equal monthly installments over four years, subject to continued services to the Company through the applicable vesting date. If a change in control occurs, 100% of such options will become fully vested.
Additionally, on August 25, 2023, we granted each of Mr. Dhillon and Ms. Arsenault (i) performance-based RSUs covering 81,110 and 53,529 shares of our common stock, respectively, and (ii) contingent performance-based RSUs covering 194,270 and 128,209 shares of our common stock, respectively, subject to approval of the Plan Amendment, which was approved by the Majority Stockholders and the Board on September 29, 2023. These RSUs vest upon achievement of the following performance milestones, subject to continued services to the Company through the applicable vesting date: 25% of the restricted stock units vest upon the Company achieving a market capitalization of $125 million, $250 million, $400 million, respectively, and an additional 25% vests if the Company achieves a value of $500 million or greater at an exit event. If the Company achieves an exit value greater than $500 million at an earlier date, subject to the executive’s continued services with the Company through such exit event, all of the RSUs will vest. No RSUs will vest until the compensation committee of the Company determines that shares can be sold into the market to cover withholding tax obligations associated with the vesting of the restricted stock units. As of December 31, 2023, no market capitalization milestone was achieved and no restricted stock units were vested.
For additional information, please see below under “Outstanding Equity Awards at Fiscal Year End.”
72
Employment and Severance Arrangements
Employment Agreement with Punit Dhillon
On August 7, 2020, we entered into an employment agreement with Mr. Dhillon, our Chief Executive Officer. The agreement provides for an annual base salary of $400,000 per year and an annual discretionary bonus up to 50% of his base salary based on Mr. Dhillon’s achievement of annual corporate milestones agreed to by the Board. Effective June 1, 2022, Mr. Dhillon's annual base salary was increased to $450,000 per year and his annual discretionary bonus eligibility was increased to 60% of his base salary. Mr. Dhillon also receives the normal benefits available to other similarly situated executives and will be entitled to severance pay under the circumstances described below.
Mr. Dhillon’s employment with the Company is at-will. The employment agreement provides that, except for a termination of Mr. Dhillon’s employment for “Cause,” “By Death”, “By Disability” (as such terms are defined in his employment agreement), Mr. Dhillon is entitled to a severance payment equal to 24 months of his then current base salary, less applicable statutory deductions and withholdings if terminated by the Company.
Employment Agreement with Kaitlyn Arsenault
On October 4, 2021, we entered into an employment agreement with Ms. Arsenault, our Chief Financial Officer. The agreement provides for an annual base salary of $300,000 per year and an annual discretionary bonus of up to 35% of her base salary based in part on Ms. Arsenault’s achievement of milestones agreed to by the Board. Effective June 1, 2022, Ms. Arsenault’s annual base salary was increased to $340,000 per year and her annual discretionary bonus eligibility was increased to 40% of her base salary. Ms. Arsenault also receives the normal benefits available to other similarly situated executives and will be entitled to severance pay under the circumstances described below.
Ms. Arsenault’s employment with the Company is at-will. The employment agreement provides that, except for a termination of Ms. Arsenault’s employment for “Cause,” “By Death” or “By Disability” (as such terms are defined in her employment agreement), (a) in the event that following a “Change of Control” (as defined in the Company's Amended and Restated 2014 Omnibus Incentive Plan) Ms. Arsenault’s employment is terminated by the Company, she will be entitled to a severance payment equal to 12 months of her then current base salary less applicable statutory deductions and withholdings, and (b) in the event that prior to a Change of Control, Ms. Arsenault’s employment is terminated by the Company, she would be entitled to a severance payment equal to (i) 6 months of her then current base salary, less applicable statutory deductions and withholdings, if such termination were to occur before April 4, 2023, (ii) 9 months of her then current base salary, less applicable statutory deductions and withholdings, if such termination were to occur on or after April 4, 2023 and before October 4, 2024, and (iii) 12 months of her then current base salary, less applicable statutory deductions and withholdings, if such termination were to occur on or after October 4, 2024.
The foregoing description of the employment agreements above does not purport to be complete and is qualified in its entirety by reference to the full text of the employment agreements attached hereto as an exhibit and incorporated by reference herein.
73
Outstanding Equity Awards at Fiscal Year-end
As of December 31, 2023, our named executive officers held the following outstanding Company equity awards:
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name | Grant Date |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Un- exercisable |
Option Exercise Price ($) |
Option Expiration Date |
Number of
Shares or Units of
Stock That Have Not
Vested (#)
|
Market
Value of
Shares or Units of Stock That Have Not
Vested ($)(1)
|
Number of
Unearned
Shares, Units or Other Rights That Have Not Vested
(#)
|
Market or Payout Value Of
Unearned
Shares, Units or Other Rights That Have Not Vested
($)
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Punit Dhillon, |
10/10/2018(2)
|
800 | — | 76.25 | 10/10/2028 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CEO/Chairman |
8/7/2020(3)
|
25,200 | 10,800 | 11.25 | 8/7/2030 | |||||||||||||||||||||||||||||||||||||||||||||||||||
12/14/2021(4)
|
6,180 | 6,180 | 14.50 | 12/14/2031 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
12/14/2021(5)
|
2,667 | 7,254 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
8/25/2023(8)
|
752 | 8,261 | 3.50 | 8/25/2033 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
9/29/2023(8)
|
1,798 | 19,788 | 3.50 | 9/29/2033 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
8/25/2023(9)
|
81,110 | 220,619 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
9/29/2023(9)
|
194,270 | 528,414 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kaitlyn Arsenault |
9/15/2021(6)
|
880 | 720 | 30.00 | 9/15/2031 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CFO |
10/4/2021(7)
|
3,520 | 2,880 | 22.50 | 10/4/2031 | |||||||||||||||||||||||||||||||||||||||||||||||||||
12/14/2021(4)
|
3,540 | 3,540 | 14.50 | 12/14/2031 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
12/14/2021(5)
|
1,333 | 3,626 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
8/25/2023(8)
|
1,116 | 12,267 | 3.50 | 8/25/2033 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
9/29/2023(8)
|
2,671 | 28,159 | 3.50 | 9/29/2033 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
8/25/2023(9)
|
53,529 | 145,599 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
9/29/2023(9)
|
128,209 | 348,728 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1)The market value of shares that have not vested is calculated based on the per share closing price of our common stock on December 31, 2023.
(2)The options specified above vest as follows: 1/12th each month on the anniversary of the grant date.
(3)The options specified above vest as follows: 10% vests on the grant date and 90% vests in equal semi-annually installments thereafter over four years.
(4)The options specified above vest as follows: 25% vests on the one year anniversary of the grant date and 1/48th vests monthly thereafter over three years following the one year anniversary of the grant date.
(5)The restricted stock units specified above vest as follows: 33% on each grant date anniversary over three years.
(6)The options specified above vest as follows: 10% vests on the grant date and 90% vests in equal annual installments thereafter over four years.
(7)The options specified above vest as follows: 10% vests on the grant date and 90% vests in equal semi-annually installments thereafter over four years.
(8)The options specified above vest as follows: monthly on the grant date thereafter over four years.
74
(9)The restricted stock units vest on the following performance milestones: an incremental 25% of the RSUs vest upon the Company achieving a market capitalization of $125 million, $250 million, $400 million, respectively, and an additional 25% vests if the Company achieves a value of $500 million or greater at an exit event. If the Company achieves an exit value greater than $500 million at an earlier date, subject to the executive’s continued services with the Company through such exit event, all of the RSUs will vest. No RSUs shall vest until the compensation committee of the Company determines that shares can be sold into the market to cover withholding tax obligations associated with the vesting of the restricted stock units. As of December 31, 2023, no market capitalization milestone was achieved and no RSUs were vested.
Exercises of Options
There were no exercises of stock options by our named executive officers during the year ended December 31, 2023.
Director Compensation
As of December 31, 2023, our policy for the compensation of our non-employee directors is as follows:
•Each non-employee director receives a cash retainer of $40,000 on an annual basis, and an executive chair of the Board, if one is appointed as such and is a non-employee director, receives an additional $40,000 retainer annually.
•Upon election to the Board, non-employee directors receive a one-time award of 20,000 stock options which vest in twelve equal monthly installments. In subsequent annual periods, each non-employee director receives a grant of 20,000 stock options which vest in twelve equal monthly installments.
Non-employee directors who serve as members of special committees of the Board receive additional compensation as follows:
•Audit Committee: $10,000 per year ($20,000 for the chair)
•Compensation Committee: $3,500 per year ($10,000 for the chair)
•Nominating and Corporate Governance Committee: $2,500 per year ($5,000 for the chair)
On January 5, 2023, each of Drs. Dalesandro, Tyle, and Ward received 1,000 options, and on February 14, 2023, Dr. Charych received 1,000 options, which options vest monthly over 12 months. These grants were made in consideration of their service as a director of the Company for the year ended December 31, 2022 and were consistent with the non-employee director compensation policy in place at the time of the grant.
On August 25, 2023, each director other than Mr. Grayson received an annual grant of 20,000 stock options, which vest monthly over 12 months. These grants were made in consideration of their service as a director of the Company for the year ended December 31, 2023 and were consistent with the non-employee director compensation policy as of December 31, 2023.
In connection with Mr. Grayson’s appointment to the Board and in recognition of his skills, experience and future contributions to the Company, the Company paid Mr. Grayson a cash bonus of $350,000 on September 18, 2023. In addition, in recognition of his skills, experience and future contributions to the Company, on August 25, 2023, we granted Mr. Grayson performance-based RSUs covering 72,531 shares of our common stock and contingent performance-based RSUs covering 173,721 shares of our common stock, subject to approval of the Plan Amendment, which was approved by the Majority Stockholders and the Board on September 29, 2023. The restricted stock units vest on the following milestones: 25% of the restricted stock units vest upon the Company achieving a market capitalization of $125 million, $250 million, $400 million, respectively, and an additional 25% vests if the Company achieves a value of $500 million or greater at an exit even or greater at an exit event. If the Company achieves an exit value greater than $500 million at an earlier date, subject to Mr. Grayson’s continued services with the Company through such exit event, all of the restricted stock units will vest. No restricted stock units will vest until the compensation committee of the Company determines that shares can be sold into the market to cover withholding tax obligations associated with the vesting of the restricted stock units. As of December 31, 2023, no market capitalization milestone was achieved and no RSUs were vested.
75
The table below summarizes the compensation paid by us to our non-employee directors for the year ended December 31, 2023. Mr. Dhillon, our employee director, does not receive additional compensation for his services as a member of our Board:
| DIRECTOR COMPENSATION | ||||||||||||||||||||||||||||||||
| Name |
Fees
Earned
or Paid
in Cash
($)
|
Stock
Awards
($) (1)
|
Option
Awards
($) (1)
|
Total ($) |
||||||||||||||||||||||||||||
Margaret Dalesandro |
54,516 | — | 61,842 | (2) |
116,358 | |||||||||||||||||||||||||||
Praveen Tyle |
58,184 | — | 61,842 | (3) |
120,026 | |||||||||||||||||||||||||||
Keith Ward |
61,504 | — | 61,842 | (4) |
123,346 | |||||||||||||||||||||||||||
Deborah Charych |
35,688 | — | 66,592 | (5) |
102,280 | |||||||||||||||||||||||||||
Andy Schwab |
15,988 | — | 58,092 | (6) |
74,080 | |||||||||||||||||||||||||||
Paul Grayson |
364,731 | (8) |
889,458 | — | (7) |
1,254,189 | ||||||||||||||||||||||||||
(1)The amounts reported under "Stock Awards" and “Option Awards” in the above table reflect the grant date fair value of these awards as determined in accordance with the Financial Accounting Standards Board’s Accounting Standards Codification Topic 718, Compensation - Stock Compensation. The value of stock option awards was estimated using the Black-Scholes option pricing model. The valuation assumptions used in the valuation of options granted may be found in Note 8 to our financial statements included in this annual report on Form 10-K for the year ended December 31, 2023. The annual Board member grants for the year ended December 31, 2023, were granted on August 25, 2023. As of December 31, 2023, each non-employee director is entitled to an annual grant of 20,000 common stock options, all of which vest in twelve equal monthly installments.
(2)The aggregate number of shares issuable upon exercise of option awards outstanding on December 31, 2023 for Dr. Dalesandro was 22,601, of which 9,267 were fully vested.
(3)The aggregate number of shares issuable upon exercise of option awards outstanding at December 31, 2023 for Dr. Tyle was 22,101, of which 8,767 were fully vested.
(4)The aggregate number of shares issuable upon exercise of option awards outstanding at December 31, 2023 for Dr. Ward was 22,001, of which 8,667 were fully vested.
(5)The aggregate number of shares issuable upon exercise of option awards outstanding at December 31, 2023 for Dr. Charych was 21,001, of which 7,501 were fully vested.
(6)The aggregate number of shares issuable upon exercise of option awards outstanding at December 31, 2023 for Mr. Schwab was 20,001, of which 6,667 were fully vested.
(7)As of December 31, 2023, Mr. Grayson had 246,252 restricted stock units with market and performance based vesting conditions. The restricted stock units vest on the following milestones: 25% of the restricted stock units vest upon the Company achieving a market capitalization of $125 million, $250 million, $400 million, respectively, and an additional 25% vests if the Company achieves a value of $500 million or greater at an exit event. If the Company achieves an exit value greater than $500 million at an earlier date, subject to Mr. Grayson’s continued services with the Company through such exit event, all of the restricted stock units will vest. No restricted stock units will vest until the compensation committee of the Company determines that shares can be sold into the market to cover withholding tax obligations associated with the vesting of the restricted stock units. As of December 31, 2023, no market capitalization milestone was achieved and no restricted stock units were vested.
(8)Amount includes the prorated annual cash retainer that Mr. Grayson received for his service from August 18, 2023 to December 31, 2023 and a cash bonus of $350,000 in connection with Mr. Grayson’s appointment to the Board and in recognition of his skills, experience and future contributions to the Company.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Securities Authorized for Issuance under Equity Compensation Plans
76
The table below includes the following information as of December 31, 2023 for the Company’s 2014 Amended and Restated Omnibus Incentive Plan (the “2014 Amended and Restated Plan”). Shares available for issuance under the 2014 Amended and Restated Plan can be granted pursuant to stock options, stock appreciation rights, restricted stock, restricted stock unit awards, performance awards and other stock-based or cash-based awards, as selected by the plan administrator. For additional information about the 2014 Amended and Restated Plan, refer to Note 8 in our consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
| Equity Compensation Plan Information | ||||||||||||||||||||
| Plan category | Number of shares of common stock to be issued upon exercise of outstanding options, warrants and rights (a) |
Weighted- average exercise price of outstanding options, warrants and rights (b) |
Number of shares of common stock remaining available for future issuance under equity compensation plans (excluding shares of common stock reflected in column (a)) (c) |
|||||||||||||||||
| Equity compensation plans approved by security holders | ||||||||||||||||||||
| 2014 Amended and Restated Omnibus Incentive Plan | 1,346,075 | $ | 8.96 | 487,672 | ||||||||||||||||
| 2022 Employee Stock Purchase Plan | — | — | 112,000 | |||||||||||||||||
Equity compensation plans not approved by security holders |
— | — | — | |||||||||||||||||
| Total | 1,346,075 | $ | — | 599,672 | ||||||||||||||||
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information with respect to beneficial ownership of our common stock as of March 20, 2024, by:
•each person known to be the beneficial owner of 5% or more of our outstanding common stock;
•each executive officer;
•each director; and
•all of the executive officers and directors as a group.
Beneficial ownership has been determined in accordance with Rule 13d-3 under the Exchange Act. Under this rule, certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire shares (for example, upon exercise of an option or warrant or vesting of an RSU) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares is deemed to include the amount of shares beneficially owned by such person by reason of such acquisition rights. As a result, the percentage of outstanding shares of any person as shown in the following table does not necessarily reflect the person’s actual voting power at any particular date.
The information set forth in the table below is based on 28,062,907 shares of our common stock issued and outstanding on March 20, 2024.
To our knowledge, except as indicated in the footnotes to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them. Unless otherwise indicated, the address of each beneficial owner listed below is 11250 El Camino Real, Suite 100, San Diego, CA 92130.
77
| Name and Address of Beneficial Owner | Beneficial Ownership |
Percent of Class |
|||||||||||||||
| More than 5% Beneficial Owners | |||||||||||||||||
Entities affiliated with 5am Ventures |
11,884,898 | (1) | 40.29 | % | |||||||||||||
Entities affiliated with Versant Ventures III LLC. |
2,530,950 | (2) | 8.86 | % | |||||||||||||
Altium Growth Fund, L.P. |
1,801,518 | (3) | 6.42 | % | |||||||||||||
Entities affiliated with Sphera Global Healthcare Management L.P. |
1,501,518 | (4) | 5.35 | % | |||||||||||||
| Named Executive Officers and Directors | |||||||||||||||||
| Punit Dhillon | 289,780 | (5) | 1.02 | % | |||||||||||||
| Kaitlyn Arsenault, CPA | 159,066 | (6) | *% | ||||||||||||||
| Tuan Tu Diep | 76,997 | (7) | *% | ||||||||||||||
| Dr. Margaret Dalesandro | 21,767 | (8) | *% | ||||||||||||||
| Dr. Praveen Tyle | 21,267 | (9) | *% | ||||||||||||||
| Dr. Keith Ward | 21,167 | (10) | *% | ||||||||||||||
| Dr. Deborah Charych | 20,167 | (11) | *% | ||||||||||||||
| Andrew Schwab | 19,167 | (12) | *% | ||||||||||||||
| Paul Grayson | 190,522 | (13) | *% | ||||||||||||||
| Dr. Annalisa Jenkins | 6,667 | (14) | *% | ||||||||||||||
| All executive officers and directors as a group (10 persons) | 826,567 | 2.9 | % | ||||||||||||||
_________
*Denotes beneficial ownership of less than 1% of our outstanding shares of common stock.
(1)Based on a Schedule 13D/A filed with the SEC on March 13, 2024, which reported that the beneficial ownership includes (i) 8,393,520 shares of Common Stock held by 5AM Ventures VII L.P. (“Ventures VII”) and (ii) 1,705,393 shares of Common Stock issuable upon exercise of warrants held by Ventures VII that are currently exercisable, (iii) 1,718,189 shares of Common Stock held by 5AM Ventures II, L.P. (“Ventures II”) and (iv) 67,796 shares held by 5AM Co-Investors II, L.P. (“Co-Investors II”). 5AM Partners VII, LLC (“Partners VII”) serves as sole general partner of Ventures VII and shares voting and dispositive power over the securities held by Ventures VII. 5AM Partners II, LLC (“Partners II”) serves as sole general partner of Ventures II and Co-Investors II. Andrew J. Schwab, Dr. Kush Parmar, Dr. John D. Diekman are managing members of Partners II. Each of Partners II, Andrew J. Schwab, Dr. Kush Parmar, Dr. John D. Diekman shares voting and dispositive power over the securities held by Ventures II and Co-Investors II. Andrew J. Schwab, one of our directors, is an affiliate of Ventures VII, Ventures II, and Co-Investors II. Each of Partners VII, Partners II, Andrew J. Schwab, Dr. Kush Parmar, Dr. John D. Diekman disclaim beneficial ownership of such shares except to the extent of its or their pecuniary interest therein. The address of all entities affiliated with Ventures VII is c/o 5AM Ventures, 501 2nd Street, Suite 350, San Francisco, CA 94107.
(2)Based on a Schedule 13G filed with the SEC on August 28, 2023, which reported that the beneficial ownership includes (i) 1,995,916 shares of Common Stock held by Versant Venture Capital III, L.P. (“Versant III”), (ii) 520,173 shares of Common Stock issuable upon exercise of warrants held by Versant Venture Capital III, L.P. that are currently exercisable, (iii) 11,788 shares of Common Stock held by Versant Side Fund III, L.P. (“Side Fund III”) and (iv) 3,073 shares of Common Stock issuable upon exercise of warrants held by Side Fund III that are currently exercisable. Versant Ventures III, LLC (“Versant Ventures III”) is the sole general partner of Versant III and Side Fund III. Versant Ventures III shares voting and investment power over the securities held by Versant III and Side Fund III and as a result may be deemed to have beneficial ownership over such securities. The address of all entities affiliated with Ventures III is c/o Versant Ventures, One Sansome Street, Suite 1650, San Francisco, CA 94104.
(3)Based on a Schedule 13G filed with the SEC on February 5, 2024 and other information available to the Company. Consists of 1,801,518 shares of common stock held by Altium Growth Fund, LP. Altium Capital Management, LP, the investment manager of Altium Growth Fund, LP, has voting and investment power over these securities. Jacob Gottlieb is the managing member of Altium Capital Growth GP, LLC, which is the general partner of Altium Growth Fund, LP. Each of Altium Growth Fund, LP and Jacob Gottlieb disclaims beneficial ownership over these securities. The principal address of Altium Capital Management, LP is 152 West 57th Street, 20th Floor, New York, NY.
78
(4)Based on a Schedule 13G filed with the SEC on February 8, 2024 and other information available to the Company. Consists of (i) 256,920 shares of Common Stock are held directly by Sphera Global Healthcare Master Fund, which has delegated its investment management authority to Sphera Global Healthcare Management LP (the "Management Company") and (ii) 1,244,598 shares of common stock are held directly by Sphera Biotech Master Fund, L.P., which has delegated its investment management authority to the Management Company. The Management Company is managed, controlled, and operated by its general partner, Sphera Global Healthcare GP Ltd., the shares of which are owned 90% by Sphera Funds Management Ltd. Their business address is 4 Itzak Sade, Building A, 29th Floor, Tel Aviv 6777504, Israel.
(5)Includes (i) 9,343 shares of common stock held by a family trust of which Mr. Dhillon is the trustee, (ii) 18,961 shares of common stock held directly by Mr. Dhillon, (iii) 1,326 shares of common stock issuable upon exercise of warrants, (iv) includes 53,616 and 206,534 shares of common stock underlying options and RSUs, respectively, that may be exercised within 60 days of March 20, 2024.
(6)Includes 20,096 and 136,303 shares of common stock underlying options and RSUs, respectively,that may be exercised within 60 days of March 20, 2024.
(7)Includes 19,706 and 54,624 shares of common stock underlying options and RSUs, respectively, that may be exercised within 60 days of March 20, 2024.
(8)Includes 21,767 shares of common stock underlying options that may be exercised within 60 days of March 20, 2024.
(9)Includes 21,267 shares of common stock underlying options that may be exercised within 60 days of March 20, 2024.
(10)Includes21,167 shares of common stock underlying options that may be exercised within 60 days of March 20, 2024.
(11)Includes 20,167 shares of common stock underlying options that may be exercised within 60 days of March 20, 2024.
(12)Includes 19,167 shares of common stock underlying options that may be exercised within 60 days of March 20, 2024.
(13)Includes 5,833 and 184,689 shares of common stock underlying options and RSUs, respectively, that may be exercised within 60 days of March 20, 2024.
(14)Includes 6,667 shares of common stock underlying options that may be exercised within 60 days of March 20, 2024.
Changes in Control
Our management is not aware of any arrangements which may result in “changes in control” as that term is defined by the provisions of Item 403(c) of Regulation S-K.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Transactions with Related Persons
Except as specified below, there have been no other transactions with related persons in the last two fiscal years, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeds the lesser of $120,000 or 1% of the average of our total assets as of December 31, 2023 and 2022, and in which any related person had or will have a direct or indirect material interest.
Compensation arrangements for our directors and Named Executive Officers are described in Item 11 of this Form 10-K under the section entitled "Executive Compensation."
79
Emerald Health Sciences
On October 5, 2018, we entered into a multi-draw credit agreement with Emerald Health Sciences, Inc. (“Sciences”), who was previously a beneficial holder of more than 5% of our capital stock, for an unsecured credit facility of up to $20,000,000 (as amended or restated from time to time, the “Credit Agreement”). On November 17, 2022, we entered into an amendment to the Credit Agreement (the “November 2022 Amendment”), pursuant to which we prepaid outstanding principal amount under the Credit Agreement, equal to $616,125, plus outstanding accrued interest of $328,737 and extended the maturity date for the loan underlying the Credit Agreement. In addition, pursuant to the November 2022 Amendment, we agreed to amend the exercise price of all of the warrants to purchase Company common stock held by Sciences to $4.25 per share. On February 16, 2023, the Company and Sciences entered in a master transaction agreement (the “Master Transaction Agreement”) pursuant to which Sciences agreed to exercise 66,566 warrants to purchase common stock of the Company (the “Warrants”) and the parties agreed that the aggregate exercise price for the Warrants of $282,906 was to be paid through a reduction in the debt owed by the Company to Sciences (the “Credit Consideration”) under the Credit Agreement. Pursuant to the terms of the Master Transaction Agreement, after the application of the Credit Consideration to the amounts owed under the Credit Agreement, Sciences agreed to convert the remaining balance of $1,597,236 owed under the Credit Agreement into 165,517 shares of common stock of the Company at a conversion price of $9.65. Following the issuance of the shares described above, the Credit Agreement was terminated in its entirety.
During the periods ended December 31, 2023 and December 31, 2022, the Company paid to Sciences nil and $616,125 in principal and interest under the Credit Agreement.
On December 14, 2022, the Company and Sciences entered into a piggyback registration rights agreement pursuant to which, among other things, the Company agreed to provide registration rights for the shares of common stock underlying the warrants to purchase Company common stock held by Sciences should the Company file a registration statement with the SEC for the purpose of effecting an offering of common stock. On August 15, 2023, Sciences waived their right to include such shares in the registration statement to be filed with the Securities and Exchange Commission in connection with the Merger and 2024 Financing (as such terms are defined below).
Jim Heppell, a director of the Company from January 2019 until May 18, 2022 was also the CEO and a board member of Sciences until March 10, 2023.
VivaCell Biotechnology España, S.L.U (formerly known as Emerald Health Biotechnology España, S.L.U.)
In January 2021 and April 2021, we entered into two separate Collaborative Research Agreements with VivaCell Biotechnology Espana, S.L.U ("VivaCell"), a research and development entity with substantial expertise in cannabinoid science and a subsidiary of Emerald Health Research, Inc. which is 100% owned by Sciences. For the years ended December 31, 2023 and 2022, we incurred $0 and $87,927, respectively, in expenses under the Collaborative Research Agreements. No amounts were due to or from VivaCell under these agreements for the year ended December 31, 2023.
On October 11, 2021, we entered into an Exclusive Sponsored Research Agreement (the “ESRA”) with VivaCell to fund certain research and development programs which are of mutual interest to both the Company and VivaCell. On May 8, 2023, the Company terminated the ESRA effective March 31, 2023 and Vivacell waived the required notice period under the ESRA.
For the years ended December 31, 2023 and 2022, we incurred $50,000 and $200,000 in expenses under the ESRA. As of December 31, 2023 and 2022, we recognized accounts payable of $0 and $50,000, respectively.
On March 1, 2022, we entered into a research project with VivaCell under the ESRA Agreement for the development of a screening platform for anteroposterior ocular diseases. The project budget is $190,500. For the year ended December 31, 2023 and 2022, we incurred $39,167 and $167,000, respectively, of research and development expenses under the ESRA. As of December 31, 2023 and 2022, we recognized $0 and $7,835, respectively, in other current liabilities - related parties related to the first research project. As of December 31, 2023 and 2022, we recognized $0 and $47,001, respectively, in accounts payable - related parties under this agreement.
Merger and 2023 Financing
On August 18, 2023, the Company completed the acquisition of Bird Rock Bio, Inc., a Delaware corporation (“Bird Rock”), in accordance with the terms of the Agreement and Plan of Merger and Reorganization, dated August 15, 2023 (the “Merger Agreement”), by and among the Company, Aquila Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of the Company (“Merger Sub”) and Bird Rock. Pursuant to the Merger Agreement, Merger Sub merged with and into Bird Rock, with Bird Rock surviving such merger as a wholly owned subsidiary of the Company (the “Merger”).
Pursuant to the Merger Agreement, at the effective time of the Merger (the “Effective Time”), the Company issued to certain former stockholders of Bird Rock, an aggregate of 3,872,184 shares of the common stock of the Company, par value $0.001 per share (the “Common Stock”), valued at approximately $20.0 million based on the 60 trading day volume weighted average price of the Common Stock as of an agreed upon date.
80
In connection with the execution of the Merger Agreement, on August 15, 2023, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain investors (collectively, the “Investors”), pursuant to which the Company sold to the Investors an aggregate of 2,325,537 shares of Common Stock (the “PIPE Shares”), at a price of $5.16 per share, and accompanying warrants to purchase up to 2,325,537 of Common Stock (the “PIPE Warrants”), for an aggregate purchase price of $12.0 million (the “2023 Financing”).
Pursuant to the Merger Agreement and the Purchase Agreement, at the Effective Time, the Company issued to certain former stockholders of Bird Rock, an aggregate of 2,228,638 shares of Common Stock as a result of the participation of such former stockholders or their respective affiliates in the 2023 Financing.
Following the consummation of the Merger and the Financing, each of 5am Ventures and Affiliates and Versant Ventures and Affiliates became owners of more than 5% of our common stock.
On August 15, 2023, in connection with the execution of the Merger Agreement and the Purchase Agreement the Company entered into a Registration Rights Agreement (the “Registration Rights Agreement”) with certain investors, pursuant to which such holders of Company securities will have certain customary registration rights, including rights with respect to the filing of a registration statement under the Securities Act within 180 days from the date of the Registration Rights Agreement.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers. The indemnification agreements, our articles of incorporation and our bylaws require us to indemnify our directors to the fullest extent not prohibited by Nevada law. Subject to certain limitations, our bylaws also require us to advance expenses incurred by our directors and officers.
Review, Approval and Ratification of Related Party Transactions
It is the Company's policy that all related party transactions must be approved by directors independent of the parties involved. All of the transactions described above were approved and ratified by the independent members of our Board. In connection with the approval of the transactions described above, our Board took into account several factors, including their fiduciary duties to the Company, the relationships of the related parties described above to the Company, the material facts underlying each transaction, the anticipated benefits to the Company and related costs associated with such benefits, whether comparable products or services were available, and the terms we could receive from an unrelated third party.
Conflicts Related to Other Business Activities
The persons serving as our officers and directors have existing responsibilities and, in the future, may have additional responsibilities, to provide management and services to other entities in addition to us. As a result, conflicts of interest between us and the other activities of those persons may occur from time to time.
We will attempt to resolve any such conflicts of interest in our favor. Our officers and directors are accountable to our shareholders and us as fiduciaries, which requires that such officers and directors exercise good faith and integrity in handling our affairs. A shareholder may be able to institute legal action on our behalf or on behalf of that shareholder and all other similarly situated shareholders to recover damages or for other relief in cases of the resolution of conflicts in any manner prejudicial to us.
Director Independence
We have determined that Dr. Margaret Dalesandro, Dr. Praveen Tyle, Dr. Keith Ward, Dr. Annalisa Jenkins and Dr. Deborah Charych are independent members of our Board, as that term is defined in Rule 5605(a)(2) of the Nasdaq Listing Rules.
Item 14. Principal Accounting Fees and Services.
Audit Fees
The aggregate fees billed for each of the fiscal years ended December 31, 2023 and 2022, for professional services rendered by Marcum LLP. for the audit of our annual consolidated financial statements included in our Annual Report on Form 10-K and quarterly reviews of the unaudited interim consolidated financial statements included in our Quarterly Reports on Form 10-Q or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for the years ended December 31, 2023 and 2022 were $285,000 and $125,861, respectively.
Audit Related Fees
None.
81
Tax Fees
None.
All Other Fees
None.
Pre-Approval Policies and Procedures
Prior to engaging Marcum LLP to perform audit services, our Board obtains an estimate for the service to be performed. All of the services described above were approved by the members of the Audit Committee of the Board in accordance with its procedures.
82
PART IV
Item 15. Exhibits, Financial Statement Schedules.
Financial Statements. The following consolidated financial statements of Skye Bioscience, Inc., together with the report thereon of Marcum LLP, an independent registered public accounting firm (PCAOB Firm No. 688 ), are included in this Annual Report on Form 10-K.
83
SKYE BIOSCIENCE, INC. AND SUBSIDIARIES INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of Skye Bioscience, Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Skye Bioscience, Inc. and Subsidiaries (the “Company”) as of December 31, 2023 and 2022, the related consolidated statements of operations, stockholders’ deficit and cash flows for each of the two years in the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provides a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ Marcum LLP
Marcum LLP
We have served as the Company's auditor since 2022.
March 21, 2024
F-2
SKYE BIOSCIENCE, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31 | |||||||||||
| ASSETS | 2023 | 2022 | |||||||||
| Current assets | |||||||||||
Cash |
$ | $ | |||||||||
| Restricted cash | |||||||||||
| Prepaid expenses | |||||||||||
| Assets held for sale | |||||||||||
| Other current assets | |||||||||||
| Total current assets | |||||||||||
| Property, plant and equipment, net | |||||||||||
Operating lease right-of-use asset |
|||||||||||
| Other assets | |||||||||||
| Total assets | $ | $ | |||||||||
LIABILITIES AND STOCKHOLDERS' DEFICIT |
|||||||||||
| Current liabilities | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accounts payable - related parties | |||||||||||
| Accrued interest - related party | |||||||||||
Accrued interest - legal contingency |
|||||||||||
| Accrued payroll liabilities | |||||||||||
| Other current liabilities | |||||||||||
| Other current liabilities - related parties | |||||||||||
| Estimate for legal contingency | |||||||||||
Convertible multi-draw credit agreement - related party |
|||||||||||
Convertible note - related party, net of discount |
|||||||||||
| Operating lease liability, current portion | |||||||||||
| Total current liabilities | |||||||||||
| Non-current liabilities | |||||||||||
| Operating lease liability, net of current portion | |||||||||||
| Total liabilities | |||||||||||
Commitments and contingencies (Note 13) |
|||||||||||
Stockholders’ deficit |
|||||||||||
Preferred stock, $ |
|||||||||||
Common stock, $ |
|||||||||||
| Additional paid-in-capital | |||||||||||
| Accumulated deficit | ( |
( |
|||||||||
Total stockholders’ deficit |
( |
( |
|||||||||
Total liabilities and stockholders’ deficit |
$ | $ | |||||||||
See accompanying notes to the consolidated financial statements. | |||||||||||
F-3
SKYE BIOSCIENCE, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year Ended December 31 | |||||||||||
| 2023 | 2022 | ||||||||||
| Operating expenses | |||||||||||
| Research and development | $ | $ | |||||||||
Cost to acquire IPR&D asset |
|||||||||||
| General and administrative | |||||||||||
| Estimated legal contingency | ( |
||||||||||
| Total operating expenses | |||||||||||
| Operating loss | ( |
( |
|||||||||
| Other expense | |||||||||||
| Change in fair value of derivative liability | ( |
( |
|||||||||
| Interest expense | |||||||||||
| Interest income | ( |
( |
|||||||||
| Finance charge | |||||||||||
| Loss from asset sale | |||||||||||
| Debt conversion inducement expense | |||||||||||
| Wind-down costs | |||||||||||
| Total other expense, net | |||||||||||
| Loss before income taxes | ( |
( |
|||||||||
| Provision for income taxes | |||||||||||
| Net loss | $ | ( |
$ | ( |
|||||||
| Loss per common share | |||||||||||
| Basic | $ | ( |
$ | ( |
|||||||
| Diluted | $ | ( |
$ | ( |
|||||||
| Weighted average shares of common stock outstanding used to compute loss per share: | |||||||||||
| Basic | |||||||||||
| Diluted | |||||||||||
See accompanying notes to the consolidated financial statements.
F-4
SKYE BIOSCIENCE, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31 | |||||||||||
| 2023 | 2022 | ||||||||||
| Cash flows from operating activities: | |||||||||||
| Net loss | $ | ( |
$ | ( |
|||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
| Finance charge from Sciences warrant modification | |||||||||||
| Depreciation and amortization | |||||||||||
Net gain on disposal of asset |
( |
||||||||||
| Stock-based compensation expense | |||||||||||
| Change in fair value of derivative liability | ( |
( |
|||||||||
Amortization of debt discount |
|||||||||||
| Estimate for legal contingency | ( |
||||||||||
Loss from divestiture of asset |
|||||||||||
| Debt conversion inducement expense | |||||||||||
| Accrued interest conversion expense | |||||||||||
| Cost to acquire IPR&D asset | |||||||||||
| Foreign currency remeasurement gain | ( |
||||||||||
| Changes in assets and liabilities: | |||||||||||
| Prepaid expenses | ( |
||||||||||
| Prepaid expenses - related party | |||||||||||
| Other current assets | ( |
||||||||||
| Accounts payable | ( |
||||||||||
| Accounts payable – related parties | ( |
||||||||||
| Accrued interest – related party | ( |
||||||||||
Accrued interest - legal contingency |
|||||||||||
| Accrued payroll liabilities | |||||||||||
| Other current liabilities | ( |
( |
|||||||||
| Other current liabilities - related parties | ( |
||||||||||
| Operating lease liability | ( |
( |
|||||||||
Net cash and restricted cash used in operating activities |
( |
( |
|||||||||
| Cash flows from investing activities: | |||||||||||
| Proceeds from asset sale, net of legal expenses | — | ||||||||||
| Cash divested net of proceeds from the sale of an asset | — | ( |
|||||||||
| Purchases of property and equipment | ( |
( |
|||||||||
Cash acquired in asset acquisition, net of transaction costs of $ |
|||||||||||
Net cash and restricted cash provided by investing activities |
|||||||||||
| Cash flows from financing activities: | |||||||||||
Proceeds from PIPE financing, net of $ |
|||||||||||
Proceeds from convertible note - related party |
|||||||||||
Financing costs allocated to warrants issued with convertible debt |
( |
||||||||||
| Proceeds from pre-funded warrant exercises | |||||||||||
| Repayment of loan payable | ( |
( |
|||||||||
| Proceeds from EHT bridge financing | |||||||||||
| Repayment of Amended Credit Agreement | ( |
||||||||||
Net cash and restricted cash provided by (used in) financing activities |
( |
||||||||||
Net increase (decrease) in cash and restricted cash |
( |
||||||||||
Cash and restricted cash, beginning of year
|
$ | $ | |||||||||
Cash and restricted cash, end of year |
$ | $ | |||||||||
F-5
| Supplemental disclosures of cash-flow information: | |||||||||||
| Reconciliation of cash and restricted cash: | |||||||||||
Cash |
$ | $ | |||||||||
| Restricted cash | |||||||||||
| Total cash and restricted cash shown in the consolidated statements of cash flows | $ | $ | |||||||||
| Cash paid during the year for: | |||||||||||
| Interest | $ | $ | |||||||||
| Income taxes | |||||||||||
| Supplemental disclosures of non-cash financing activities: | |||||||||||
| Financing of insurance premium | $ | $ | |||||||||
| Common stock warrant exercises | |||||||||||
| Conversion of multi-draw credit agreement | |||||||||||
| Conversion of accrued interest due to related party | |||||||||||
| Right of use asset obtained in exchange for operating lease liabilities | |||||||||||
| Stock issued for assets | — | ||||||||||
| Deferred issuance costs | |||||||||||
| Purchases of property and equipment in other current liabilities | |||||||||||
| Release of share liability to additional paid-in-capital | |||||||||||
| Asset acquisition costs in other current liabilities and accounts payable | |||||||||||
| Stock issued for assets, net of equity issuance costs | — | ||||||||||
F-6
SKYE BIOSCIENCE, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' DEFICIT
Stockholders' Deficit |
|||||||||||||||||||||||||||||
| Common Stock | Additional Paid-In Capital |
Accumulated Deficit |
Total
Stockholders'
Deficit
|
||||||||||||||||||||||||||
| Shares | Amounts | ||||||||||||||||||||||||||||
| Balance, December 31, 2021 |
|
$ |
|
$ |
|
$ | ( |
$ |
|
||||||||||||||||||||
| Stock-based compensation expense | — | ||||||||||||||||||||||||||||
Exercise of pre-funded warrants |
— | ||||||||||||||||||||||||||||
Common stock, options and warrants issued for asset acquisition, net of issuance costs of $ |
— | ||||||||||||||||||||||||||||
| Finance charge from Sciences warrant modification | — | — | |||||||||||||||||||||||||||
Net loss for the year ended December 31, 2022 |
— | — | — | ( |
( |
||||||||||||||||||||||||
| Balance, December 31, 2022 |
|
$ |
|
$ |
|
$ | ( |
$ | ( |
||||||||||||||||||||
| Stock-based compensation expense | — | ||||||||||||||||||||||||||||
Exercise of common stock warrants |
— | ||||||||||||||||||||||||||||
Conversion of multi-draw credit agreement - related party and accrued interest |
— | ||||||||||||||||||||||||||||
Common stock issued in acquisition of IPR&D asset |
— | ||||||||||||||||||||||||||||
PIPE Financing, net of equity issuance costs $ |
— | ||||||||||||||||||||||||||||
| Warrants issued with Convertible Note | — | — | — | ||||||||||||||||||||||||||
Common stock issued for fractional share adjustment in reverse stock split |
( |
— | |||||||||||||||||||||||||||
Net loss for the year ended December 31, 2023 |
— | — | — | ( |
( |
||||||||||||||||||||||||
| Balance, December 31, 2023 |
|
$ |
|
$ |
|
$ | ( |
$ | ( |
||||||||||||||||||||
See accompanying notes to the consolidated financial statements.
F-7
SKYE BIOSCIENCE, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of Operations and Business Activities
Nature of Operations
Skye Bioscience, Inc. (the “Company” or “Skye”) was incorporated in Nevada on March 16, 2011. The Company is a clinical stage pharmaceutical company located in San Diego, California, focused on the discovery, development and commercialization of novel classes of therapeutic drugs that modulate the endocannabinoid system, which has been shown to play a vital role in overall human health. Notably, the Company is developing drugs with novel mechanisms of action targeting the CB1 receptor through its own research efforts acquired intellectual property and license agreements.
In August 2019, the Company formed a new subsidiary in Australia, SKYE Bioscience Pty Ltd. (formerly "EMBI Australia Pty Ltd."), an Australian proprietary limited company ("SKYE Bioscience Australia"), in order to qualify for the Australian government’s research and development tax credit for research and development dollars spent in Australia. The Company conducted its Phase 1 clinical trial for glaucoma at SKYE Bioscience Australia.
On August 18, 2023, the Company completed a strategic transaction to acquire a clinical asset pursuant to an Agreement and Plan of Merger and Reorganization, dated as of August 15, 2023, by and among the Company, Bird Rock Bio, Inc. and Aquila Merger Sub, Inc., pursuant to which Aquila Merger Sub, Inc. merged with and into Bird Rock Bio, Inc. with Bird Rock Bio, Inc. surviving as a wholly owned subsidiary of the Company (the “BRB Acquisition”). In connection with the BRB Acquisition, Bird Rock Bio changed its name from Bird Rock Bio, Inc. to Bird Rock Bio Sub, Inc ("BRB"). In the BRB Acquisition, the Company issued to certain former stockholders of BRB an aggregate of 5,436,378 shares of the common stock of the Company, valued at $21,609,586 (Note 3).
As of December 31, 2023, the Company has devoted substantially all its efforts to securing product licenses, carrying out its own research and development, building infrastructure and raising capital. The Company has not yet realized revenue from its planned principal operations and is a number of years away from potentially being able to do so.
Liquidity and Capital Resources
The Company has incurred operating losses and negative cash flows from operations since inception and as of December 31, 2023, had a working capital deficit of $2,250,156 and an accumulated deficit of $104,382,549 . As of December 31, 2023, the Company had unrestricted cash in the amount of $1,256,453 . For the years ended December 31, 2023 and 2022, the Company incurred losses from operations of $34,735,173 and $18,311,732 , respectively. For the years ended December 31, 2023 and 2022, the Company incurred net losses of $37,644,784 and $19,481,602 , respectively. The Company expects to continue to incur significant losses and negative cash flows from operations through 2024 and in the future.
Historically, the Company has funded its operations through convertible debt, public equity financings, asset acquisitions and private investments in public equity. On August 18, 2023, the Company entered into the Convertible Note Financing, the August PIPE Financing and BRB Acquisition which provided the Company with the necessary funds to continue operations post an appeal bond to stay the execution of the judgment in the Cunning Lawsuit (Note 13) and reposition the Company to focus on nimacimab as its lead clinical asset for obesity. Following the August 2023 financings, the Company executed a 1:250 reverse stock split and increased its authorized shares outstanding (Note 7). On January 31, 2024 and March 13, 2024, the Company completed two private placement equity transactions with institutional investors, in which it raised combined net aggregate proceeds of approximately $83,500,000 . The capital from the January and March financings will allow the Company to fund its planned Phase 2 clinical trials for glaucoma and obesity through top line data.
The Company’s consolidated financial statements have been prepared on the basis of the Company continuing as a going concern for the next 12 months. Based on its current operational requirements, the Company believes that its current cash, and cash equivalents will be sufficient to fund its projected operations for at least 12 months from the date of the issuance of these consolidated financial statements.
Impact of Geopolitical and Macroeconomic Factors
It is possible that the Company may encounter supply chain issues related to global economic and political conditions such as a lack of production or laboratory resources. pandemics or cyberattacks that could cause business disruptions and clinical trial delays which will need to be managed in the future. There may also be significant uncertainty resulting from the impact of other geopolitical and macroeconomic factors, including global pandemics, inflation, supply chain issues, rising interest rates, future bank failures, increased geopolitical tensions between the U.S. and China and the impact of the Russia/Ukraine conflict and the Israel-Hamas war.
F-8
2. Summary of Significant Accounting Policies
Basis of Presentation
The preparation of financial statements in conformity with U.S. Generally Accepted Accounting Principles ("GAAP") requires management to make estimates and assumptions that affect the amounts reported in the Consolidated Financial Statements and the accompanying notes. Actual results could differ from those estimates.
Certain reclassifications have been made to the amounts in prior periods to conform to the current period’s presentation, primarily the separate classification of prepaid expenses, other current assets, estimate for legal contingency, accrued interest for legal contingency, and other current liabilities. Such reclassifications did not have a material impact on the Consolidated Financial Statements.
Reverse Stock Split
On September 6, 2023, the Company filed a Certificate of Change and Certificate of Correction with the Secretary of State of the State of Nevada which effected a reverse stock split at a ratio of one-for-two hundred and fifty (1-for-250) of the Company's issued and outstanding shares of common stock as of 12:01 a.m. Eastern Standard Time on September 8, 2023. The Company did not issue fractional shares in the reverse stock split and elected to issue one whole share for each fractional share which resulted in the issuance of 26,349 common shares to our existing stockholders. The Company's financial statements have been adjusted on a retrospective basis to reflect the change.
Assets Held for Sale
On November 10, 2022, the Company completed the EHT Acquisition. At the time of the EHT Acquisition there were arrangements in place to sell the acquired assets and liabilities that comprised two of EHT's subsidiaries, Emerald Health Therapeutics Canada, Inc. ("EHTC") and VDL. As a result, EHTC and VDL were considered held for sale since the EHT Acquisition and the Company has classified the associated assets of VDL as held for sale on the Consolidated Balance Sheets and the period costs related to both EHTC and VDL have been presented as wind-down costs in the Consolidated Statements of Operations. EHTC was divested on December 28, 2022, and VDL was divested on February 9, 2023 (see Note 3). Assets meeting the held-for-sale criteria are classified as held for sale on the Consolidated Balance Sheets in subsequent periods until sold.
Assets that meet the held-for-sale criteria are held for sale and reported at the lower of their carrying value or their fair value, less estimated costs to sell. Changes in fair value are recorded as a gain or loss in the results of operations but not to exceed the original carrying value. Due to the asset acquisition accounting on the date of the EHT Acquisition, AVI had no initial carrying value.
Derecognition of Nonfinancial Assets
The Company generally accounts for sales of nonfinancial assets that are outside the scope of our ordinary activities under ASC 610-20, Other Income - Gains and Losses from the Derecognition of Nonfinancial Assets. Pursuant to ASC 610-20, the Company applies the guidance in ASC 606 to determine if a contract exists, identify the distinct nonfinancial assets, and determine when control transfers and, therefore, when to derecognize the nonfinancial asset. Additionally, the Company applies the measurement principles of ASC 606 to determine the amount of consideration, if any, to include in the calculation of the gain or loss for the sale of the nonfinancial asset. Refer to Note 3 for further information.
Principles of Consolidation
The accompanying consolidated financial statements as of December 31, 2023, include the accounts of the Company and its wholly owned subsidiaries SKYE Bioscience Australia, EHT, AVI, BRB, Ruiyi Acquisition Corporation, and Nemus Sub. All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of the Consolidated Financial Statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements and the reported amounts of income and expense during the reporting period. Actual results could differ from those estimates. The most significant accounting estimates inherent in the preparation of the Company’s financial statements include estimates and judgements used in determining stock based compensation expense and estimates related to the Company's estimation of the percentage of completion under its research and development contracts, which are not readily apparent from other sources.
F-9
Risks and Uncertainties
The Company’s operations are subject to a number of risks and uncertainties, including but not limited to, changes in the general economy, the size and growth of the potential markets for any of the Company’s product candidates, uncertainties related to the current global environment, including economic factors such as inflation, and risks related to the global supply chain disruptions (Note 1), risks related to operating primarily in a virtual environment, results of research and development activities, uncertainties surrounding regulatory developments in the United States, Canada, the European Union, and Australia and the Company’s ability to attract new funding.
Cash, Cash Equivalents and Restricted Cash
The Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. The carrying values of those investments approximate their fair value due to their short maturity and liquidity. Cash includes cash on hand and amounts on deposit with financial institutions, which amounts may at times exceed federally insured limits. The Company has not experienced any losses on such accounts and does not believe it is exposed to any significant credit risk.
Property, Plant and Equipment, net
Fair Value Measurements
Certain assets and liabilities are carried at fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (the “exit price”) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. A fair value hierarchy based on three levels of inputs, of which the first two are considered observable, and the last is considered unobservable, is used to measure fair value:
Level 1: Valuations for assets and liabilities traded in active markets from readily available pricing sources such as quoted prices in active markets for identical assets or liabilities.
Level 2: Observable inputs (other than Level 1 quoted prices) such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data.
Level 3: Unobservable inputs that are supported by little or no market activity and that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques.
The carrying values of the Company’s financial instruments, with the exception of the derivative liabilities, approximate their fair value due to their short maturities. The derivative liabilities are valued on a recurring basis utilizing Level 3 inputs (Note 5).
Income Taxes
The Company accounts for deferred income tax assets and liabilities based on differences between the financial reporting and tax bases of assets and liabilities, net operating loss carryforwards (the “NOLs”) and other tax credit carryforwards. These items are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the period that includes the enactment date. Any interest or penalties would be recorded in the Company’s Consolidated Statements of Operations in the period incurred. When necessary, the Company recognizes interest and penalties related to income tax matters in income tax expense.
F-10
The Company records a valuation allowance against deferred tax assets to the extent that it is more likely than not that some portion or all of the deferred tax assets will not be realized. In making such determinations, management considers all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax planning strategies and recent financial operations. Due to the substantial doubt related to the Company’s ability to utilize its deferred tax assets, a valuation allowance for the full amount of the deferred tax assets has been established at December 31, 2023 and 2022. As a result of this valuation allowance, there are no income tax benefits reflected in the accompanying Consolidated Statements of Operations to offset pre-tax losses.
The Company recognizes a tax benefit from uncertain tax positions when it is more likely than not (50%) that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits of the position.
Convertible Instruments
The Company accounts for hybrid contracts with embedded conversion features in accordance with ASC 815, Derivatives and Hedging Activities ("ASC 815") which requires companies to bifurcate conversion options from their host instruments and account for them as free-standing derivative financial instruments according to certain criteria. The criteria includes circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under otherwise applicable GAAP with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument.
The Company accounts for convertible debt instruments with embedded conversion features in accordance with ASC 470-20, Debt with Conversion and Other Options ("ASC 470-20") if it is determined that the conversion feature should not be bifurcated from their host instruments. Under ASC 470-20, the Company records, when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the difference between the fair value of the underlying common stock at the commitment date and the embedded effective conversion price. When the Company determines that the embedded conversion option should be bifurcated from its host instrument, the embedded feature is accounted for in accordance with ASC 815. Under ASC 815, a portion of the proceeds received upon the issuance of the hybrid contract is allocated to the fair value of the derivative. The derivative is subsequently recorded at fair value at each reporting date based on current fair value, with the changes in fair value reported in the results of operations.
The Company also follows ASC 480-10, Distinguishing Liabilities from Equity ("ASC 480-10") when evaluating the accounting for its hybrid instruments. A financial instrument that embodies an unconditional obligation, or a financial instrument other than an outstanding share that embodies a conditional obligation, that the issuer must or may settle by issuing a variable number of its equity shares shall be classified as a liability (or an asset in some circumstances) if, at inception, the monetary value of the obligation is based solely or predominantly on any one of the following: (a) a fixed monetary amount known at inception (for example, a payable settled with a variable number of the issuer’s equity shares); (b) variations in something other than the fair value of the issuer’s equity shares (for example, a financial instrument indexed to the Standard and Poor’s S&P 500 Index and settled with a variable number of the issuer’s equity shares); or (c) variations inversely related to changes in the fair value of the issuer’s equity shares (for example, a written put option that could be net share settled). Hybrid instruments meeting these criteria are not further evaluated for any embedded derivatives and are carried as a liability at fair value at each balance sheet date with a re-measurement reported in other expense (income), net in the accompanying Consolidated Statements of Operations.
When determining the short-term vs. long-term classification of derivative liabilities, the Company first evaluates the instruments’ exercise provisions. Generally, if a derivative is a liability and exercisable within one year, it will be classified as short-term. However, because of the unique provisions and circumstances that may impact the accounting for derivative instruments, the Company carefully evaluates all factors that could potentially restrict the instrument from being exercised or create a situation where exercise would be considered remote. The Company re-evaluates its derivative liabilities at each reporting period end and makes updates for any changes in facts and circumstances that may impact classification.
Warrants Issued in Connection with Financings
The Company generally accounts for warrants issued in connection with debt and equity financings as a component of equity, unless the warrants include a conditional obligation to issue a variable number of shares or there is a deemed possibility that the Company may need to settle the warrants in cash. For warrants issued with a conditional obligation to issue a variable number of shares or the deemed possibility of a cash settlement, the Company records the fair value of the warrants as a liability at each balance sheet date and records changes in fair value in other expense, net in the Consolidated Statements of Operations.
F-11
Debt Issuance Costs and Interest
Discounts related to bifurcated derivatives, freestanding instruments issued in bundled transactions, and issuance costs are recorded as a reduction to the carrying value of the debt and amortized over the life of the debt using the effective interest method. The Company makes changes to the effective interest rate, as necessary, on a prospective basis. For debt facilities that provide for multiple advances, the Company initially defers any issuance costs until the first advance is made and then amortizes the costs over the life of the facility.
Revenue Recognition
The Company accounts for its collaboration arrangement under the provisions of Accounting Standard Codification Topic 606, Revenue from Contract with Customers, or ASC 606. In accordance with ASC 606, when a customer obtains control of promised goods or services, in an amount that reflects the consideration expected to be received in exchange for those goods or services, the Company performs the following five steps in determining the appropriate amount of revenue to be recognized as it fulfills its obligations under such agreements:
•identification of the promised goods and services in the contract;
•determination of whether the promised goods or services are performance obligations including whether they are distinct in the context of the contract;
•measurement of the transaction price, including any constraint on variable consideration;
•allocation of the transaction price to the performance obligations; and
•recognition of revenue when, or as, we satisfy each performance obligation.
If an agreement includes a license to the Company's intellectual property and that license is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenues allocated to the license when the license is transferred to the customer and the customer is able to use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgment to assess the nature of the combined performance obligations to determine whether the combined performance obligations are satisfied over time or at a point in time. If over time, the Company evaluates the measure of progress over time proportionate to the costs incurred to perform the services using an input method as a measure of progress towards satisfying the performance obligation. Any change made to estimated progress towards completion of a performance obligation due to changes in the estimated activities required to complete the performance obligation and, therefore, revenue recognized will be recorded as a change in estimate.
The Company receives payments from its collaborators based on billing schedules established in each contract. Upfront payments and other payments may require deferral of revenue recognition to a future period until the Company performs its obligation under its collaboration arrangements. Amounts are recorded as accounts receivable when the Company’s right to consideration is unconditional.
Research and Development Expenses and Licensed Technology
Research and development costs are expensed when incurred. These costs may consist of external research and development expenses incurred under agreements with third party contract research organizations and investigative sites; third party manufacturing organizations and consultants; license fees; employee-related expenses, which include salaries and benefits for the personnel involved in the Company’s preclinical; and clinical drug development activities, other expenses and equipment and laboratory supplies.
Costs incurred for the rights to use licensed technologies in the research and development process, including licensing fees and milestone payments, are charged to research and development expense as incurred in situations where the Company has not identified an alternative future use for the acquired rights, and are capitalized in situations where there is an identified alternative future use. None of the costs associated with the use of licensed technologies has been capitalized to date.
Similarly, costs incurred to acquire in-process research and development ("IPR&D") are charged to research and development expense in the situation where the Company has not identified an alternative future use and are capitalized in the situation where there is an alternative future use. All costs associated with the acquisition of IPR&D have been expensed to date.
Stock-Based Compensation Expense
Stock-based compensation expense is estimated at the grant date based on the fair value of the award, and the fair value is recognized as expense ratably over the vesting period with forfeitures accounted for as they occur.
F-12
Upon the exercise of stock option awards, the Company's policy is to issue new shares of its common stock. The Company uses the Black-Scholes valuation method for estimating the grant date fair value of stock options using the following assumptions:
•Volatility - Stock price volatility is estimated over the expected term based on a blended daily rate of industry peers stock volatility.
•Expected term - The expected term is based on a simplified method which defines the life as the weighted average of the contractual term of the options and the vesting period for each award.
•Risk-free rate - The risk-free interest rate for the expected term of the option is based on the average market rate on U.S. Treasury securities in effect during the period in which the awards were granted.
•Dividends - The dividend yield assumption is based on the Company’s history and expectation of paying no dividends in the foreseeable future.
Loss Per Common Share
Leases
The Company applies ASU, No. 2016-02, Leases (Topic 842), in accounting for operating lease arrangements.
At the inception of an arrangement, the Company determines whether the arrangement is, or contains, a lease based on the unique facts and circumstances present. Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of lease payments over the expected lease term. The interest rate implicit in the lease contract is typically not readily determinable. As such, the Company utilizes its incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment. Certain adjustments to the right-of-use asset may be required for items such as initial direct costs paid or incentives received.
Lease expense is recognized over the expected term on a straight-line basis. Operating leases are recognized on the Consolidated Balance Sheets as operating lease right-of-use assets, operating lease liability, current portion and operating lease liability, net of current portion.
Asset Acquisitions
The Company evaluates acquisitions of assets and other similar transactions to assess whether or not the transaction should be accounted for as a business combination or asset acquisition by first applying a screen test to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets. If the screen is met, the transaction is accounted for as an asset acquisition. If the screen is not met, further determination is required as to whether or not the Company has acquired inputs and processes that have the ability to create outputs which would meet the definition of a business. Significant judgment is required in the application of the screen test to determine whether an acquisition is a business combination or an acquisition of assets.
F-13
Government Assistance
The Company adopted ASU 2021-10 Government Assistance on January 1, 2022. The Company accounts for the tax rebates received from the Australian Taxation Office ("ATO") under such guidance. The Company accounts for the rebates that it receives under the AusIndustry research and development tax incentive program under the income recognition model of IAS 20. Under this model, when there is reasonable assurance that the rebate will be received, the Company recognizes the income from the tax rebate as an offset to research and development expense during the period which the benefit applies to the research and development costs incurred. The total tax rebates received under the AusIndustry incentive program were $180,374 for the year ended December 31, 2023 related to incentives earned in the prior year and $34,189 for the year ended December 31, 2022. As of December 31, 2023 and 2022, the Company has recognized $540,604 and $179,687 , respectively, in other current assets in its Consolidated Balance Sheets.
Foreign Currency Translation
The Company’s reporting currency and the functional currency of its foreign subsidiaries is the United States dollar. The local currencies of its foreign subsidiaries are the Canadian Dollar (“CAD”) or Australian dollar (“AUD”). Assets and liabilities are remeasured based on the exchange rates at the balance sheet date 0.7549 for the CAD, 0.6818 for the AUD as of December 31, 2023 and 0.7384 for the CAD and 0.6792 for the AUD as of December 31, 2022, while expense accounts are remeasured at the weighted average exchange rate for the period 0.7453 for the CAD and 0.6697 for the AUD for the year ended December 31, 2023 and 0.7361 for the CAD and 0.6748 for the AUD as of December 31, 2022. Equity accounts are remeasured at historical exchange rates. The resulting remeasurement adjustments are recognized in general and administrative expenses in the consolidated financial statements.
During the years ended December 31, 2023 and 2022, the Company recorded foreign currency remeasurements of $61,767 and $63,717 , respectively, which are reflected in general and administrative expenses in the accompanying Consolidated Statements of Operations.
Commitments and Contingencies
The Company follows ASC 440, Commitments and ASC 450, Contingencies, subtopic 450-20 to report accounting for contingencies and commitments respectively. Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur.
The Company assesses such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or un-asserted claims that may result in such proceedings, the Company evaluates the perceived merits of any legal proceedings or un-asserted claims as well as the perceived merits of the amount of relief sought or expected to be sought therein.
F-14
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potentially material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, and an estimate of the range of possible losses, if determinable and material, would be disclosed. Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the guarantees would be disclosed. Based upon information available at this time, management believes that the current litigation matter related to the Cunning lawsuit will have a material adverse effect on the Company’s consolidated financial position, results of operations and cash flows. Refer to Note 13 for additional information.
In accordance with ASC 450, Contingencies, subtopic 450-20, the Company does not reflect a contingency that may result in a gain until it is realized.
Recent Accounting Pronouncements
Recently Adopted Accounting Pronouncements
In June 2016, the Financial Accounting Standards Board (“FASB”) issued Account Standards Update (“ASU”) No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, which requires the measurement and recognition of expected credit losses for financial assets held at amortized cost. This ASU replaces the existing incurred loss impairment model with an expected loss model. It also eliminates the concept of other-than-temporary impairment and requires credit losses related to available-for-sale debt securities to be recorded through an allowance for credit losses rather than as a reduction in the amortized cost basis of the securities. These changes will result in earlier recognition of credit losses. The amendments in this ASU are effective for the Company for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. The Company adopted ASU 2016-13 as of January 1, 2023 and the adoption did not have a material impact on the Company’s consolidated financial statements and related disclosures.
Recent Accounting Pronouncements Not Yet Adopted
In December 2023, the FASB issued ASU 2023-09, Improvements to Income Tax Disclosures. This ASU requires greater disaggregation of information about a reporting entity's effective tax rate reconciliation as well as information on income taxes paid. This ASU applies to all entities subject to income taxes and is intended to help investors better understand an entity’s exposure to potential changes in jurisdictional tax legislation and assess income tax information that affects cash flow forecasts and capital allocation decisions. This ASU is effective for annual periods beginning after December 15, 2024, with early adoption permitted. This ASU should be applied on a prospective basis although retrospective application is permitted. The Company is currently evaluating the impact the adoption of this ASU will have on its consolidated financial statements and related disclosures
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. The amendments in this ASU require disclosures, on an annual and interim basis, of significant segment expenses that are regularly provided to the chief operating decision maker (“CODM”), as well as the aggregate amount of other segment items included in the reported measure of segment profit or loss. This ASU requires that a public entity disclose the title and position of the CODM and an explanation of how the CODM uses the reported measure(s) of segment profit or loss in assessing segment performance and deciding how to allocate resources. Public entities will be required to provide all annual disclosures currently required by Topic 280 in interim periods, and entities with a single reportable segment are required to provide all the disclosures required by the amendments in this ASU and existing segment disclosures in Topic 280. This ASU is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The amendments in this ASU should be applied retrospectively to all prior periods presented in the financial statements. The Company is currently evaluating the impact of this standard on its consolidated financial statements and related disclosures, and does not expect the standard will have a material impact on the Company’s consolidated financial statements and related disclosures.
In August 2020, the FASB issued ASU 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. This ASU amends the guidance on convertible instruments and the derivatives scope exception for contracts in an entity’s own equity and improves and amends the related EPS guidance for both Subtopics. The ASU will be effective for annual reporting periods beginning after December 15, 2023 and interim periods within those annual periods and early adoption is permitted in fiscal periods ending after December 15, 2020. Upon implementation, the Company may use either a modified retrospective or full retrospective method of adoption. The adoption of ASU 2020-06 will, result in expanded disclosures around convertible instruments and remove the requirement to assess and record beneficial conversion features. The Company currently plans to adopt the provisions of this ASU on the effective date using a modified retrospective method of adoption.
F-15
3. Asset Acquisitions
BRB Acquisition
On August 18, 2023, the Company acquired 100 % of Bird Rock Bio Sub, Inc. pursuant to an Agreement and Plan of Merger and Reorganization, dated August 15, 2023. The purpose of the acquisition was to acquire BRB's clinical asset, nimacimab, an antibody targeting the CB1 receptor, for development to treat obesity. Pursuant to the BRB Acquisition, the Company issued 3,872,184 shares of Skye common stock to the former preferred shareholders of BRB equal to $20,000,000 in base merger consideration priced at $5.16 .
In addition, the former preferred shareholders of BRB were entitled to additional merger consideration for each dollar invested in the August 2023 PIPE Financing (Note 7). Because the August 2023 PIPE Financing and BRB Acquisition occurred contemporaneously and in contemplation of each other, in accounting for the transaction, the Company allocated the shares issued as additional merger consideration between the BRB Acquisition and PIPE Financing using a residual allocation method, whereby the fair value of the consideration transferred was first allocated to the monetary assets and August 2023 PIPE Financing proceeds with the remainder allocated to the IPR&D asset. As a result, 1,564,194 additional shares of common stock were allocated to the BRB Acquisition.
Below is a summary of the total consideration, assets acquired and the liabilities assumed in connection with the BRB Acquisition:
| August 18, 2023 | |||||||||||
| Purchase consideration | |||||||||||
| Common stock | $ | (a) | |||||||||
| Total consideration | $ |
|
|||||||||
| Assets acquired and liabilities assumed: | |||||||||||
IPR&D asset |
$ | ||||||||||
| Cash and cash equivalents | |||||||||||
| Prepaid expenses | |||||||||||
| Accounts payable | ( |
||||||||||
| Other current liabilities | ( |
||||||||||
| Total net assets acquired | $ |
|
|||||||||
(a) Equal to the aggregate common shares issued of 5,436,378 , multiplied by the Company's closing stock price of $3.975 as of August 18, 2023.
The cost to acquire the IPR&D asset related to nimacimab was expensed on the date of the BRB Acquisition as it was determined to have no future alternative use. Accordingly, costs associated with the BRB Acquisition to acquire the asset were expensed as incurred.
Acquisition of Emerald Health Therapeutics, Inc.
On May 11, 2022, the Company entered into an Arrangement Agreement, as amended on June 14, 2022, July 15, 2022 and October 14, 2022 (the “Arrangement Agreement”) with Emerald Health Therapeutics, Inc., a corporation existing under the laws of the Province of British Columbia, Canada (“EHT”), pursuant to a plan of arrangement under the Business Corporations Act (British Columbia) (the “EHT Acquisition”). On November 10, 2022, the Company completed the EHT Acquisition and each share of EHT common stock outstanding immediately prior to the effective time of the EHT Acquisition was transferred to the Company in exchange for 1.95 shares of the Company's common stock (the “Exchange Ratio”).
The Company evaluated the accounting for the transaction and accounted for the EHT Acquisition as an asset acquisition due to the wind-down state of EHT. The primary purpose of the EHT Acquisition was to utilize EHT's remaining cash and cash equivalents and liquidate the primary real estate asset owned by EHT in order to fund the Company's operations. To account for the Acquisition, the Company measured the equity interests issued on the Closing Date (including the value of the options and warrants rolled over) and accumulated the direct costs attributable to the Acquisition.
F-16
Upon closing the EHT Acquisition, the Company acquired net assets with an estimated fair value of $15,045,412 . The fair value of the consideration was allocated on a relative fair value basis to the “qualifying assets” in the EHT Acquisition and any excess in the fair value of the assets initially reduced the value of the qualifying assets before reducing the value of the assets held for sale. The only qualifying asset identified in the EHT Acquisition was AVI. The fair value of AVI at the time of Acquisition was $1,536,275 and the value attributable to AVI was fully eliminated in the Acquisition accounting . As of December 31, 2023 the Company has divested both of EHT's former operating entities and as of January 15, 2024, the divestiture of substantially all of EHTs assets, including the real estate held by AVI, is complete.
Upon the Closing Date of the EHT Acquisition, the Company issued each EHT shareholder 1.95 shares of Skye common stock, for each share of EHT common stock outstanding as of the Closing Date. On November 10, 2022, the Company issued 1,665,083 shares of stock as consideration in the EHT Acquisition and no fractional shares of Skye Common Stock were issued. For U.S. and Canadian federal income tax purposes, the EHT Acquisition constitutes a taxable exchange by the EHT shareholders. In addition, all outstanding stock options and warrants of EHT were exchanged for replacement options and warrants of Skye with identical terms, as adjusted in accordance with the Exchange Ratio.
Below is a summary of the total consideration, assets acquired and the liabilities assumed in connection with the Acquisition:
| November 10, 2022 | |||||||||||
| Purchase consideration | |||||||||||
| Common stock | $ | (a) | |||||||||
| EHT rollover stock options | (b) | ||||||||||
| EHT rollover warrants | (c) | ||||||||||
| Transaction costs | (d) | ||||||||||
| Total consideration | $ |
|
|||||||||
| Assets acquired and liabilities assumed: | |||||||||||
| Cash and cash equivalents | $ | ||||||||||
| Accounts receivable | |||||||||||
| Prepaid Expenses | |||||||||||
| Assets held for sale | (e) | ||||||||||
| Related party loan | (f) | ||||||||||
| Other current assets | (g) | ||||||||||
| Accounts payable | ( |
||||||||||
| Short term liability | ( |
(h) | |||||||||
| Payroll liabilities | ( |
||||||||||
| Insurance premium loan payable | ( |
||||||||||
| Tax liabilities | ( |
||||||||||
| Other current liabilities | ( |
(i) | |||||||||
| Total net assets acquired | $ |
|
|||||||||
a.Common Stock, The Company issued 1,665,083 shares of common stock at $5.75 per share for an aggregate fair value of $9,574,222 .
b.EHT Rollover Stock Options, The estimated fair value of options issued as consideration in the EHT Acquisition was $105,929 and 33,132 SKYE options were issued after applying the Exchange Ratio. The assumptions to value these options were as follows (see Note 8):
| November 10, 2022 | |||||
| Dividend yield | |||||
| Volatility | |||||
| Risk-free interest rate | |||||
| Expected term (years) | |||||
F-17
c.EHT Rollover Warrants, The estimated fair value of warrants issued as consideration for the Acquisition was $203,515 and 243,781 SKYE warrants were issued after applying the Exchange Ratio.
The assumptions used to value these warrants are as follows:
| November 10, 2022 | ||||||||
| Dividend yield | ||||||||
| Volatility | ||||||||
| Risk-free interest rate | ||||||||
| Expected term (years) | ||||||||
d.Transaction Costs, The Company incurred aggregate transaction costs of $1,945,140 in connection with the Acquisition, of which $341,629 were expensed, $1,552,490 were considered part of the transaction consideration and $25,511 , represented equity issuance costs, which were included as an offset to equity.
e.Assets held for sale, The Company acquired assets related to EHT and its subsidiaries which are considered held for held for sale in the amount of $6,610,662 . This amount is primarily composed of the following balances:
i.The adjusted fair value of the VDL assets held for sale of $8,540,732 , net of direct liquidation costs of $390,241 , which includes legal costs, advisory fees and other professional fees. In addition, the VDL assets were further reduced by $2,072,981 as a result of the relative fair value allocation. The resulting carrying value of the asset recorded by the Company is $6,467,751 .
ii.The Company acquired deposits related to utilities for EHT's subsidiaries held for sale. The fair value of these deposits at the time of acquisition is $23,910 .
iii.The Company has acquired the value of EHTC's Health Canada license which was transferred with the sale of EHTC (See Divestiture of Emerald Health Therapeutics Canada, Inc. below). The value of the license at the time of the acquisition was $91,700 .
iv.The Company acquired prepaid expenses related to entities held for sale of $27,301 .
f.Related party loan, on October 17, 2022, the Company and EHT entered into a loan agreement pursuant to which EHT loaned the Company $700,000 in accordance with the terms of a promissory note. Upon closing the Acquisition, the loan was offset by the balance due to Skye under the consulting agreement. The net related party loan balance was $680,901 as of the closing of the Acquisition. After the closing of the EHT Acquisition, this balance eliminates in consolidation.
g.Other current assets, The Company acquired other current assets related to EHT and its subsidiaries which are considered held for held for sale in the amount of $356,961 . This amount is primarily composed of the following balances:
i.The Company acquired deposits related to EHT's excise tax bonds of $252,418 . As a condition of the EHTC and VDL stock purchase agreements it is expected that the cash value of these bonds will be received upon transfer of the Health Canada licenses to the purchasers of EHTC and VDL.
ii.The Company acquired an open receivables balance of $104,543 made up of a balance due from the buyer of VDL, a former customer of EHT's of $75,396 . Additionally, this balance includes a property tax refund due of $29,147 .
h.Short-term liability EHT received an upfront deposit of $557,010 for the sale of VDL,
i.Other current liabilities, The Company acquired liabilities related to EHT and its subsidiaries which are considered in the amount of $722,839 . This amount is primarily composed of the following balances:
i.The Company acquired an outstanding accrued liabilities balance of $587,139 . The majority of the balance includes estimated late fees related to late tax filings.
ii.In accordance with ASC 450, the Company has recorded a contingent liability related credits due to customers of EHT's former operations. At the time of the EHT Acquisition, this liability was estimated at $135,700 .
F-18
Divestiture of Emerald Health Therapeutics Canada, Inc.
On December 28, 2022, approximately six weeks after the EHT Acquisition, the Company entered into a Share Purchase Agreement (“SPA”) with a third-party whereby the Company transferred all of its outstanding and fully paid, non-assessable 11,776,338 shares of common stock (the "EHTC Common Shares"), all of which were held by EHT with no par value, for the total purchase price of $110,759 . The purchase price also includes the transfer of two licenses issued by Health Canada. EHTC was classified as an asset acquisition and did not meet the criteria of a business at the of EHT Acquisition, and was considered held for sale at the time of EHT Acquisition. Therefore, the sale of EHTC is determined to be treated as the sale of an asset to a third-party due to the discontinued state of the business at the date of divestment. No gain or loss related to the divestiture of EHTC was recorded.
Divestiture of VDL
On November 10, 2022, EHT and C3, a third-party, entered into the Verdélite SPA, as amended, effective November 8, 2022, pursuant to which C3 would acquire all of the outstanding shares of VDL, the holder of EHT's most significant real estate asset.
On February 9, 2023, upon closing the transactions contemplated by the Verdélite SPA, the Company sold all of the outstanding shares of VDL for an aggregate purchase price of approximately $9,451,233 . Prior to closing the EHT Acquisition, EHT received a $557,705 cash deposit, which was considered in the sale as of the closing date. Upon closing, the Company received gross proceeds, net of legal and advisory fees as of the closing date, of $5,532,266 . The remainder of the purchase price will be paid as follows: (i) $370,350 will be payable in five (5 ) equal monthly installments payable on the last day of each month beginning on December 31, 2023, and ending April 30, 2024, with interest in accordance with the terms of the Verdélite SPA and (ii) $2,777,625 will be payable in three (3) equal installments on each of the 18-month , 30-month , and 42-month anniversaries of the VDL Closing Date, with interest in accordance with the terms of the Verdélite SPA. The Company recognized the sale of VDL when control transferred on February 9, 2023. In accordance with recognition guidance, the Company has determined to fully reserve for the remaining receivables and will record a gain on the sale when additional cash payments are received. For the year ended December 31, 2023, the Company has recorded a loss on sale of asset of $307,086 in other expense based on the difference between the carrying amount of the assets sold and the net cash proceeds.
4. Prepaid Expenses, Other Current Assets and Other Current Liabilities
Prepaid expenses consist of the following:
| As of December 31 | |||||||||||
| 2023 | 2022 | ||||||||||
| Prepaid clinical expenses | $ | $ | |||||||||
| Total other prepaid expenses | |||||||||||
| $ |
|
$ |
|
||||||||
Other current assets consist of the following:
| As of December 31 | |||||||||||
| 2023 | 2022 | ||||||||||
| AusIndustry incentive | $ | $ | |||||||||
| Vendor deposits | |||||||||||
| Excise Tax Bonds | |||||||||||
| Other tax receivables | |||||||||||
Other current assets |
|||||||||||
| $ |
|
$ |
|
||||||||
F-19
Other current liabilities consist of the following:
| As of December 31 | |||||||||||
| 2023 | 2022 | ||||||||||
| Research and development costs | $ | $ | |||||||||
Legal expenses |
|||||||||||
EHT Acquisition related liabilities |
|||||||||||
Consulting fees |
|||||||||||
Professional fees |
|||||||||||
| Insurance loan payable | |||||||||||
Deposit - Verdélite SPA |
|||||||||||
Other accrued liabilities |
|||||||||||
| $ |
|
$ |
|
||||||||
5. Warrants and Derivative Liabilities
There are significant judgements and estimates inherent in the determination of the fair value of the Company’s warrants. These judgements and estimates include assumptions regarding the Company’s future operating performance and the determination of the appropriate valuation methods. If the Company had made different assumptions, the fair value of the warrants could have been significantly different (See Note 2).
Warrants
Warrants vested and outstanding as of December 31, 2023 are summarized as follows:
| Source | Exercise Price |
Remaining Term
(Years)
|
Number of Warrants Outstanding |
|||||||||||||||||
| 2015 Common Stock Warrants | ||||||||||||||||||||
| 2016 Common Stock Warrants to Service Providers | ||||||||||||||||||||
| 2019 Common Stock Warrants | ||||||||||||||||||||
| 2020 Common Stock Warrants to Placement Agent | ||||||||||||||||||||
| 2021 Inducement Warrants | ||||||||||||||||||||
| 2021 Inducement Warrants to Placement Agent | ||||||||||||||||||||
| 2021 Common Stock Warrants | ||||||||||||||||||||
| 2021 Common Stock Warrants to Placement Agent | ||||||||||||||||||||
| 2022 Common Stock Warrants to Service Provider | ||||||||||||||||||||
| November 2019 EHT Common Stock Warrants* | ||||||||||||||||||||
| December 2019 EHT Common Stock Warrants* | ||||||||||||||||||||
| February 2020 EHT Common Stock Warrants* | ||||||||||||||||||||
| August 2023 Convertible Note Common Stock Warrants | ||||||||||||||||||||
| August 2023 PIPE Financing Common Stock Warrants | ||||||||||||||||||||
| Total warrants outstanding as of December 31, 2023 |
|
|||||||||||||||||||
*Replacement warrants issued on November 10, 2022 in conjunction with the Acquisition (see Note 3).
As of December 31, 2023, all of the Company's warrants are fully vested.
F-20
August 2023 PIPE Financing Common Stock Warrants
In connection with the PIPE Financing (Note 7), the Company issued 2,325,537 common stock warrants. The warrants were equity classified at issuance and $4,784,894 of the gross proceeds from the PIPE Financing were allocated to the common stock warrants on a relative fair value basis. The warrants vested immediately and the fair value of $7,881,972 was determined using the Black-Scholes Merton option pricing model with the following assumptions:
| August 18, 2023 |
||||||||
| Dividend yield | % | |||||||
| Volatility factor | % | |||||||
| Risk-free interest rate | % | |||||||
| Expected term (years) | ||||||||
| Underlying common stock price | $ | |||||||
August 2023 Convertible Note Common Stock Warrants
In connection with the Convertible Note (See Note 6), the Company issued 340,000 common stock warrants. The warrants were equity classified at issuance and $931,576 of the gross proceeds from the Convertible Note were allocated to the common stock warrants on a relative fair value basis. The warrants vested immediately and the fair value of $1,144,886 was determined using the Black-Scholes Merton option pricing model with the following assumptions:
| August 18, 2023 |
||||||||
| Dividend yield | % | |||||||
| Volatility factor | % | |||||||
| Risk-free interest rate | % | |||||||
| Expected term (years) | ||||||||
| Underlying common stock price | $ | |||||||
February 2023 Sciences Warrant Exercises
Effective February 16, 2023, Company and Sciences entered into a Master Transaction Agreement (the "MTA"). Under the MTA, Sciences agreed to exercise 66,566 common stock warrants at $4.25 per share (the "MTA Warrants"). Under the MTA, the parties agreed that the aggregate proceeds from the exercise of the MTA Warrants of $282,906 was to be paid through a reduction of the Amended Credit Agreement owed by the Company to Sciences (Note 6). On February 22, 2023, the Company issued 66,566 shares of common stock to Sciences in connection with the exercise of the MTA Warrants (Note 7).
November 2022 Sciences Warrant Repricing
On November 17, 2022, the Company entered into an Amendment and Acknowledgement Agreement (the "Amendment Agreement") with Sciences. Under the terms of the Amendment Agreement, the exercise prices of all the outstanding Sciences Multi-Draw Credit Agreement Warrants and the December 2019 EHT Common Stock Warrants were repriced to $4.25 . Refer to Note 6 for further information on the Amendment Agreement.
F-21
The Company accounted for the repricing of the warrants as a modification by comparing the fair value of the warrants immediately before and after the modification date to determine the incremental fair value of the repricing. The aggregate modified fair value of $150,851 resulted in an increase in fair value of $120,228 . The Company recorded the incremental fair value as a finance charge to other expense in the Consolidated Statements of Operations for the year ended December 31, 2023. On the date of modification, the Company revalued the warrants with a Black-Scholes valuation method using the following assumptions as of the repricing date:
| November 17, 2022 | |||||
| Dividend yield | |||||
| Volatility factor | |||||
| Risk-free interest rate | |||||
| Expected term (years) | |||||
| Underlying common stock price | $ |
||||
EHT Rollover Warrants
On November 10, 2022, the Company issued equity classified replacement warrants with a fair value of $203,515 in exchange for all outstanding warrants of EHT adjusted in accordance with the Exchange Ratio. The replacement warrants were exchanged with identical terms, including exercise prices, vest terms, and expiration dates (see Note 3).
2022 Common Stock Warrants Issued to a Service Provider
On April 1, 2022, the Company granted 8,000 equity classified warrants with a fair value of $35,688 to a service provider at an exercise price of $10.00 per share. The warrants vest monthly over one year and expire on April 1, 2024. Refer to Note 8 for the summary of stock-based compensation expense.
As of the date of grant, the Company valued the warrants with a Black-Scholes valuation method using the following assumptions:
| April 1, 2022 Date of Issuance | |||||
| Dividend yield | % | ||||
| Volatility factor | % | ||||
| Risk-free interest rate | % | ||||
| Expected term (years) | |||||
| Underlying common stock price | $ | ||||
Derivative Liability
During the year ended December 31, 2023, the warrant shares underlying the Emerald Financing Warrant Liability expired unexercised and the decrease in fair value during the year ended December 31, 2023 was nominal.
The following table summarizes the activity of the derivative liability for the period indicated:
| Year Ended December 31, 2022 | |||||||||||||||||||||||||||||
| December 31, 2021 , Fair Value of Derivative Liabilities |
Fair Value of Derivative Liabilities Issued |
Change in
Fair value of
Liability
|
Reclassification of Derivatives to Equity
|
December 31, 2022, Fair Value of Derivative Liabilitiy |
|||||||||||||||||||||||||
| Emerald Financing - warrant liability | ( |
||||||||||||||||||||||||||||
| $ |
|
$ |
|
$ | ( |
$ |
|
$ |
|
||||||||||||||||||||
Emerald Financing Warrant Liability
The Emerald Financing Warrants were issued during 2018 in connection with the Emerald Financing, and originally contained a price protection feature. In connection with the August 2020 Financing, the exercise price was permanently set to $25.00 . The warrants contain a contingent put option if the Company undergoes a subsequent financing that results in a change in control. The warrant holders also have the right to participate in subsequent financing transactions on an as-if converted basis.
F-22
The Company reviewed the warrants for liability or equity classification under the guidance of ASC 480-10, Distinguishing Liabilities from Equity, and concluded that the warrants should be classified as a liability and re-measured to fair value at the end of each reporting period. The Company also reviewed the warrants under ASC 815, Derivatives and Hedging/Contracts in Entity’s Own Equity, and determined that the warrants also meet the definition of a derivative. With the assistance of a third party valuation specialist, the Company valued the warrant liabilities utilizing the Monte Carlo valuation method pursuant to the accounting guidance of ASC 820-10, Fair Value Measurements. Beginning March 31, 2021, the Company changed its valuation model for the Emerald Financing Warrant Liability to a Black-Scholes valuation method, as it was determined that a more simplistic model such as the Black-Scholes valuation method yields a substantially similar result as a Monte Carlo simulation due to the Company's current assumptions.
The warrant liability is valued at the balance sheet dates using the following assumptions:
| December, 31, 2022 | |||||
| Dividend yield | % | ||||
| Volatility factor | % | ||||
| Risk-free interest rate | % | ||||
| Expected term (years) | |||||
| Underlying common stock price | $ | ||||
6. Debt
The Company’s convertible debt consists of the following:
| As of December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
Total principal value of convertible note - related party, net of debt discount |
$ | $ | ||||||||||||
Total principal value of convertible multi-draw credit agreement - related party |
||||||||||||||
| Unamortized debt discount | ( |
|||||||||||||
| Unamortized debt issuance costs | ( |
|||||||||||||
| Carrying value of total convertible debt—related party | $ |
|
$ |
|
||||||||||
Convertible Note - Related Party
On August 15, 2023, the Company entered into a Secured Note and Warrant Purchase Agreement with MFDI, LLC (“MFDI”), pursuant to which the Company issued to MFDI a $5,000,000 secured convertible promissory note (the "Convertible Note") and a warrant to purchase 340,000 shares of common stock on August 18, 2023 (the "Convertible Note Financing") (Notes 5 & 12). The Convertible Note bears interest at a rate of 10 % per annum and matures on August 18, 2024, unless earlier repurchased or converted. The Convertible Note may be converted at any time and the conversion price is fixed at $5.16 . Accrued interest will be payable quarterly within 30 days of the last day of each calendar quarter. The Company may prepay the principal or interest outstanding under the Note at any time without penalty.
In accounting for the Convertible Note, the Company allocated $4,068,424 in proceeds to the debt host and $931,576 in proceeds to the freestanding warrants based on relative fair value. The debt discounts of $931,576 and $26,316 related to the warrants, and debt issuance costs, respectively, are being amortized over the term of the Convertible Note using the effective interest rate method. Amortization of the debt discount is recognized as non-cash interest expense in Other expense within the Consolidated Statements of Operations. In addition, the Company recorded $6,026 in equity issuance costs as a deduction to additional paid in capital in the Statements of Stockholders' Deficit.
For the year ended December 31, 2023, the effective interest rate on the Convertible Note was 31.39 %, the remaining amortization period on the debt was 0.63 years and the fair value of the underlying conversion shares did not exceed the carrying value of the debt at December 31, 2023.
F-23
Bridge Loan
On July 24, 2023, the Company entered into a loan agreement in the principal amount of $250,000 (the “Bridge Loan”) with MFDI, LLC. The Bridge Loan was obtained in order to provide bridge financing for the operations of the Company until it completed the BRB Acquisition. Concurrent with the closing of the BRB Acquisition, August 2023 PIPE Financing and Convertible Note Financing, the Bridge Loan was cancelled and converted into an investment in the August 2023 PIPE Financing (Note 7). All interest and rights related to the Bridge Loan were concurrently cancelled.
Multi-Draw Credit Agreement- Related Party
On October 5, 2018, the Company entered into the Credit Agreement with Sciences, a related party (Note 12). Between April 29, 2020 and March 29, 2021, the Company and Sciences entered into a series of Amendments until the disbursement line was closed on September 15, 2021 (the "Amended Credit Agreement"). The amendments were considered a modifications for accounting purposes.
On November 17, 2022, the Company entered into Amendment No. 4 with Sciences. Under the terms of Amendment No. 4, the parties agreed that the Company would prepay 25 % of the outstanding principal amount equal to $616,125 , plus all accrued interest of $328,737 through the date of the Amendment No. 4. In addition, the Amended Credit Agreement was amended to extend the maturity date to the earlier of December 30, 2022, or the Termination Date (as such term is defined in the Credit Agreement) and the parties agreed to use good faith efforts to enter into a customary piggyback registration rights agreement. In exchange for the extension, the Company agreed to reprice all of the outstanding Sciences warrants to $4.25 per share (Note 5).
On December 30, 2022, the Company entered into Amendment No. 5 to the Amended Credit Agreement to extend the maturity date to the earlier of (a) business days after the closing of the sale of VDL (b) February 28, 2023 or (c) the Termination Date (as such term is defined in the Amended Credit Agreement). The terms of the Amended Credit Agreement provided that convertible advances and unpaid interest may be converted into common stock at the applicable fixed conversion price of the underlying advance, subject to customary adjustments for stock splits, stock dividends, recapitalizations, etc. Effective February 16, 2022, upon entering the MTA, the remaining principal balance plus accrued interest was offset by the aggregate exercise price of $282,906 from the exercise of the MTA Warrants (Note 5) and the Company induced conversion by reducing the conversion price of the Amended Credit Agreement from $100.00 to $9.65 . The remaining balance of $1,597,236 was converted into 165,517 shares of common stock of the Company. In connection with the induced conversion, the Company recorded a debt conversion inducement expense of $1,383,285 equal to the fair value of the incremental shares issued upon conversion.
Following the issuance of shares described above, the Amended Credit Agreement was terminated in its entirety per the terms of the MTA. Additionally, under the MTA, Sciences agreed to use its best efforts to transfer all of the common stock of the Company held by Sciences to its shareholders on a pro-rata basis at or immediately prior to the Company's listing to a nationally recognized stock exchange, subject to compliance with applicable securities laws.
For the years ended December 31, 2023 and 2022, the effective interest rate related to the convertible portion of the Amended Credit Agreement was 0.00 % and 29.20 %, respectively. As of December 31, 2022, the debt discount on the convertible advances was fully amortized.
Insurance premium loan payable
On February 28, 2023, the Company entered into an annual financing arrangement for a portion of its Directors and Officers Insurance Policy (the “D&O Insurance”) with First Insurance Funding in an amount of $203,884 . The loan is payable in equal monthly installments of $23,374 , matures on January 31, 2024, and bears interest at a rate 4.24 % per annum. As of December 31, 2023 a total of $21,238 remains in prepaid expenses and the loan has been repaid.
On February 28, 2022, the Company entered into an annual financing arrangement for a portion of its Directors and Officers Insurance Policy with First Insurance Funding in an amount of $275,537 . The loan is payable in equal monthly installments of $31,150 , matures on January 31, 2023 and bears interest at a rate 4.17 % per annum. As of December 31, 2022, a total of $22,961 remains in prepaid expenses and the loan has been repaid.
F-24
Interest Expense
The Company’s interest expense consists of the following:
| Year Ended December 31, |
|||||||||||
| 2023 | 2022 | ||||||||||
| Related party interest expense – stated rate | $ | $ | |||||||||
| Insurance premium loan payable – stated rate | |||||||||||
Legal judgment interest expense |
|||||||||||
Bond premium |
|||||||||||
Premium on irrevocable letter of credit |
|||||||||||
| Other interest expense | |||||||||||
| Non-cash interest expense: | |||||||||||
| Amortization of debt discount | |||||||||||
| Amortization of transaction costs | |||||||||||
| $ |
|
$ |
|
||||||||
7. Stockholders’ Equity and Capitalization
The Company reserved shares of common stock, on an as-if converted basis, for issuance as follows:
| Year Ended December 31, |
|||||||||||
| 2023 | 2022 | ||||||||||
| Options issued and outstanding | |||||||||||
| Awards available for grant under the 2014 Plan | |||||||||||
| Shares for issuance under our equity incentive plan | |||||||||||
| Restricted stock unit awards issued and outstanding | |||||||||||
| Unreleased restricted stock awards issued to a service provider | |||||||||||
| Common stock underlying the Amended Credit Agreement | |||||||||||
| Common stock underlying the Convertible Note - Related Party | |||||||||||
| Warrants issued and outstanding | |||||||||||
Increase to Authorized Shares of Capital Stock
On November 6, 2023, the Company increased its authorized shares of common stock to 100,000,000 .
Common Stock Issuance
BRB Acquisition
On August 18, 2023, the Company issued an aggregate of 5,436,378 shares of common stock in connection with the BRB Acquisition (Note 3).
F-25
August 2023 PIPE Financing
Concurrent with the BRB Acquisition and Convertible Note Financing, on August 15, 2023, the Company entered into the August 2023 PIPE Financing, pursuant to which on August 18, 2023, the Company issued an aggregate of 2,989,981 shares of common stock and accompanying warrants to purchase up to 2,325,537 shares of common stock (the "August 2023 PIPE Financing Common Stock Warrants" - Note 5) for an aggregate purchase price of $12,000,000 . The August 2023 PIPE Financing was priced at $5.16 per share based on the 60-day volume-weighted average share price preceding August 15, 2023. The two lead investors in the PIPE Financing were also former preferred shareholders of BRB. As an incentive to participate in the August 2023 PIPE Financing, the Agreement and Plan of Merger and Reorganization with BRB entitled each BRB stockholder participating in the August 2023 PIPE Financing an additional share of common stock for every share of common stock purchased in the PIPE Financing. As a result, the two former BRB preferred shareholders who participated in the August 2023 PIPE Financing were issued an additional 2,228,638 shares of common stock. Because the PIPE Financing and BRB Acquisition occurred contemporaneously and in contemplation of one another, the Company allocated 664,444 of the common shares issued in the BRB Acquisition to the August 2023 PIPE Financing (Note 3).
In connection with the August 2023 PIPE Financing, the Company incurred $265,053 in direct equity issuance costs for net proceeds of $11,734,947 .
Stock Issued for Services
On November 1, 2023, the Company released 5,000 shares of common stock to a service provider (Note 8).
On March 2, 2022, the Company released 600 shares of common stock to a service provider (Note 8).
EHT Acquisition
On November 10, 2022, the Company issued 1,665,083 shares of common stock to EHT shareholders at a 1.95 conversion rate as consideration in the EHT Acquisition (Note 3).
Warrant Exercises
During the December 31, 2023, 66,566 of the outstanding stock warrants held by Sciences in conjunction with the MTA, with an intrinsic value of $332,830 were exercised in exchange for 66,566 shares of common stock for proceeds of $282,906 which were applied to the balance of the Amended Credit Agreement (Note 6).
During the year ended December 31, 2022, 78,667 pre-funded warrants with an intrinsic value of $1,178,033 were exercised in exchange for 78,667 shares of common stock for proceeds of $1,967 . As of December 31, 2023 all of the pre-funded warrants from the September 2021 Financing have been exercised.
Induced Conversion of Amended Credit Agreement
During the year ended December 31, 2023, the Company issued 165,517 shares of common stock to Sciences. The shares were issued in conjunction with the MTA, in exchange for the remaining principal balance plus accrued interest less the aggregate exercise price of $282,905 from the exercise of the MTA Warrants in the amount of $1,597,236 at a conversion price of $9.65 (Note 6).
Restricted Stock Units Released
On December 14, 2023, the Company released 5,333 restricted stock units that had vested to executives of the Company (Note 8).
On December 14, 2022, the Company released 5,333 restricted stock units that had vested to executives of the Company (Note 8).
8. Stock-Based Compensation
Stock Incentive Plan
On October 31, 2014, the Board approved the Company’s 2014 Omnibus Incentive Plan (the “2014 Plan”). The 2014 Plan authorizes the issuance of awards including stock options, stock appreciation rights, restricted stock, stock units and performance units to employees, directors, and consultants of the Company.
F-26
On June 14, 2022, the Board approved the 2014 Amended and Restated Omnibus Incentive Plan (the “2014 Amended and Restated Plan”) which replaced the 2014 Plan in its entirety. The 2014 Amended and Restated Plan, among other things, fixed the number of shares that can be issued under the plan to 364,879 , provided that each January 1 beginning in 2023 and ending on (and including) January 1, 2032 the number of shares will increase by 5 % of the outstanding shares of Common Stock as of the prior December 31, unless the Board of Directors of the Company decides to a lesser increase.
On September 30, 2022, the Amended and Restated 2014 Plan was approved by the shareholders. The 2014 Amended and Restated Plan authorizes the issuance of awards including stock options, stock appreciation rights, restricted stock, stock units and performance units to employees, directors, and consultants of the Company.
On September 29, 2023, the Board and Majority Stockholders adopted and approved Amendment No. 1 to the 2014 Amended and Restated Plan. Amendment No. 1 to the 2014 Amended and Restated Plan became effective on November 6, 2023. The 2014 Amended and Restated Plan was amended to increase the number of shares of the Company’s common stock authorized for issuance under the Plan by 1,299,297 to an aggregate pool of 1,846,883 , while retaining the automatic share replenishment feature.
The Company has reserved shares for issuance under our equity incentive plan upon share option exercise. As of December 31, 2023, the Company had 487,672 shares available for future grant under the 2014 Plan.
As of December 31, 2023, the shares available for future grant under the 2014 Amended and Restated Plan are as follows:
| Shares Available for Grant | |||||
| Available as of December 31, 2022 |
|
||||
| Share pool increase | |||||
| Forfeited | |||||
| Cancelled | |||||
| RSU releases | |||||
| RSU grants | ( |
||||
| Option grants | ( |
||||
| Available as of December 31, 2023 |
|
||||
Stock Options
Options granted under the 2014 Amended and Restated Plan expire no later than ten years from the date of grant. Options granted under the 2014 Amended and Restated Plan may be either incentive or non-qualified stock options. For incentive and non-qualified stock option grants, the option price shall be at least 100 % of the fair value on the date of grants, as determined by the Company’s Board of Directors. If at any time the Company grants an option, and the optionee directly or by attribution owns stock possessing more than 10 % of the total combined voting power of all classes of stock of the Company, the option price shall be at least 110 % of the fair value and shall not be exercisable more than five years after the date of grant.
Options granted under the 2014 Amended and Restated Plan may be immediately exercisable if permitted in the specific grant approved by the Board of Directors and, if exercised early may be subject to repurchase provisions. The shares issued generally vest over a period of to four years from the date of grant.
F-27
The following is a summary of option activities under the Company’s 2014 Amended and Restated Plan for the year ended December 31, 2023:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value* |
||||||||||||||||||||
| Outstanding, December 31, 2022 |
|
$ |
|
$ |
|
||||||||||||||||||
Granted |
|||||||||||||||||||||||
| Forfeited | ( |
||||||||||||||||||||||
| Cancelled | ( |
||||||||||||||||||||||
| Outstanding, December 31, 2023 |
|
$ |
|
$ |
|
||||||||||||||||||
| Exercisable, December 31, 2023 |
|
$ |
|
$ |
|
||||||||||||||||||
| Vested and expected to vest, December 31, 2023 |
|
$ |
|
$ |
|
||||||||||||||||||
*The aggregate intrinsic value is the sum of the amounts by which the quoted market price of the Company’s stock exceeded the exercise price of the stock options at December 31, 2023 for those stock options for which the quoted market price was in excess of the exercise price ("in-the-money options").
The weighted-average grant-date fair value of stock options granted for the years ended December 31, 2023 and 2022, excluding EHT rollover options issued related to the EHT Acquisition, was $2.95 and $10.00 , respectively. The total fair value of the stock options that vested during the years ended December 31, 2023 and 2022 was $512,470 and $466,263 , respectively.
The fair value of each stock option grant was estimated on the date of grant using the Black-Scholes option-pricing model under the following assumptions:
| Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Dividend yield | % | % | |||||||||
| Risk-free interest rate | |||||||||||
| Expected term (years) | |||||||||||
| Volatility | |||||||||||
In connection with the EHT Acquisition, the Company issued a total of 33,131 stock options to EHT option holders on November 10, 2022 (Note 3). The exercise price and rollover option shares were adjusted by the Exchange Ratio at the Acquisition date and retain the vest periods as originally issued.
Restricted Stock Units
On December 14, 2021, the Company granted restricted stock units (“RSUs”) to its executive management team. The RSUs cliff vest 33 % per year on the anniversary of the grant date over a three year period.
On August 25, 2023, the Company granted RSUs to its executive management team and to certain members of the Board with market and performance based conditions. The RSUs are eligible to vest subject to the achievement and attainment of certain market capitalization target goals (market-based conditions) or the achievement of a successful exit (a performance-based condition); provided, however, that no RSUs shall vest until the Compensation Committee of the Board determines that shares can be sold into the market to cover withholding tax obligations associated with the vesting of the RSUs.
F-28
| Number of Shares |
Weighted Average Grant Date Fair Value | ||||||||||
| Unvested, December 31, 2022 |
|
$ |
|
||||||||
| Granted | |||||||||||
| Released | ( |
||||||||||
| Unvested, December 31, 2023 |
|
$ |
|
||||||||
The Company used the Monte Carlo Simulation model to evaluate the derived service period and fair value of awards with market and performance conditions, including assumptions of historical volatility and risk-free interest rate commensurate with the vesting term.
The fair value of the Company's performance-based RSUs were estimated on the date of grant under the following assumptions:
| Year Ended December 31, 2023 | |||||
| Dividend yield | |||||
| Volatility factor | |||||
| Risk-free interest rate | |||||
Derived service periods (years) |
|||||
During the first quarter of 2024, the first three market based conditions of the RSUs were met.
Awards Granted Outside the 2014 Amended and Restated Plan
During the year ended December 31, 2023, the Company granted shares of common stock to a non-employee consultant for investor relations services. Half of the shares were issued upon entering each service contract and the remaining half will be issued on October 31, 2024, unless the agreement is earlier terminated.
The following is a summary of restricted stock activity outside of the 2014 Amended and Restated Plan during the year ended December 31, 2023:
| Number of Shares | Weighted Average Grant Date Fair Value | ||||||||||
| Unvested, December 31, 2022 |
|
$ |
|
||||||||
| Granted | |||||||||||
| Released | ( |
||||||||||
| *Unvested, December 31, 2023 |
|
$ |
|
||||||||
F-29
Stock-Based Compensation Expense
The Company recognizes stock-based compensation expense using the straight-line method over the requisite service period. The Company recognized stock-based compensation expense, including compensation expense for warrants with vesting provisions issued to a service provider (Note 5), and the RSUs discussed above, in its Consolidated Statements of Operations as follows:
| Year Ended December 31, |
|||||||||||
| 2023 | 2022 | ||||||||||
| Research and development | $ | $ | |||||||||
| General and administrative | |||||||||||
| $ |
|
$ |
|
||||||||
The total amount of unrecognized compensation cost was $3,955,054 as of December 31, 2023. This amount will be recognized over a weighted-average period of 7.19 years.
2022 Employee Stock Purchase Plan
9. Loss Per Share of Common Stock
The following tables are a reconciliation of the numerators and denominators used in the calculation of basic and diluted net loss per share computations:
| For the Year Ended December 31, 2023 | |||||||||||||||||
| Loss (Numerator) | Shares (Denominator) | Per-Share Amount | |||||||||||||||
| Net loss | $ | ( |
|||||||||||||||
| Basic EPS and diluted EPS | |||||||||||||||||
| Net loss available to common stockholders | ( |
$ | ( |
||||||||||||||
| For the Year Ended December 31, 2022 | |||||||||||||||||
| Income (Numerator) | Shares (Denominator) | Per-Share Amount | |||||||||||||||
| Net loss | $ | ( |
|||||||||||||||
| Basic EPS and diluted EPS | |||||||||||||||||
| Net loss available to common stockholders | ( |
$ | ( |
||||||||||||||
F-30
The following outstanding shares of common stock equivalents were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been anti-dilutive:
| Year Ended December 31, |
|||||||||||
| 2023 | 2022 | ||||||||||
| Stock options | |||||||||||
| Unvested restricted stock units | |||||||||||
Unvested restricted stock (service provider) |
|||||||||||
| Common shares underlying convertible debt | |||||||||||
| Warrants | |||||||||||
| Total | |||||||||||
10. Income Taxes
The components of loss before the income tax provision consist of the following:
| Year Ended December 31, |
|||||||||||
| 2023 | 2022 | ||||||||||
| United States | $ | ( |
$ | ( |
|||||||
| Foreign | ( |
( |
|||||||||
| Pre-tax loss and comprehensive loss from operations | $ | ( |
$ | ( |
|||||||
The components of the income tax expense consisted of the following:
| Year Ended December 31, | |||||||||||
| Current income tax expense | 2023 | 2022 | |||||||||
| Federal | $ | $ | |||||||||
| State | |||||||||||
| Foreign | |||||||||||
| Total current income tax expense | $ | $ | |||||||||
The Company is subject to taxation in the United States, various states, Australia, and Canada. The Company’s tax years for 2020 (federal), 2019 (States), 2019 (Australia) and 2019 (Canada) and forward are subject to examination by the United States, state, Australian, and Canadian tax authorities. However, to the extent allowed by law, the taxing authorities may have the right to examine periods where NOLs and credits were generated and carried forward and make adjustments up to the amount of the NOL and credit carryforwards. The Company is not currently under examination by any jurisdiction.
At December 31, 2023, the Company had federal and state NOLs aggregating $110,288,344 and $113,806,359 , respectively. If not used, $46,622,953 of Federal NOLs and $113,678,291 of state NOLs will begin to expire in 2031, $63,665,391 of federal NOLs and $128,068 of state NOLs will carry forward indefinitely subject to an 80% limitation against taxable income. At December 31, 2023, the Company had Australia NOLs aggregating $233,321 which do not expire and $43,762,031 of Canadian NOLS which begin to expire in 2024.
At December 31, 2023, the Company had Canadian capital loss carryforwards of approximately $64,743,505 which may be carried forward indefinitely.
At December 31, 2023, the Company had federal and California research credit carryforwards of $3,480,111 and $2,073,709 , respectively. The federal research credit carry forwards will begin to expire in 2027, unless previously utilized. The California research credits will carry forward indefinitely. The Company’s NOLs and research credit carryforwards are subject to a reserve. Additionally, the Company had Canadian SR&ED credits as of December 31, 2023 of $940,180 which may be carried forward indefinitely.
F-31
Utilization of the domestic NOL's and research credits could be subject to a substantial annual limitation due to ownership change limitations that may have occurred, or that could occur in the future, as required by Section 382 and 383 of the Internal Revenue Code of 1986, as amended (the Code), as well as similar state provisions. These ownership changes may limit the amount of NOLs and credits that can be utilized annually to offset future taxable income and tax, respectively. In general, an “ownership change” as defined by Section 382 of the Code, results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50 percentage points of the outstanding stock of a company by certain stockholders.
Upon the occurrence of an ownership change under Section 382 as outlined above, utilization of the NOLs and credits are subject to an annual limitation under Section 382 of the Code, which is determined by first multiplying the value of the Company’s stock at the time of the ownership change by the applicable long-term, tax-exempt rate, and then could be subject to additional adjustments, as required. Any limitation may result in expiration of a portion of the NOLs and credits before utilization. While the Company has not performed a Section 382 study, multiple ownership changes may have already occurred as the Company raised capital through the issuance of stock. However, due to the existence of the valuation allowance for deferred tax assets, any potential change in ownership will not impact the Company’s effective tax rate.
The tax effects of temporary differences and carryforwards that give rise to significant portions of the deferred income tax assets are as follows:
| As of December 31, | |||||||||||
| Current deferred tax assets and (liabilities): | 2023 | 2022 | |||||||||
| Net operating loss | $ | $ | |||||||||
| Capital loss carryforwards | |||||||||||
| Contingent legal accrual | |||||||||||
| Depreciation | |||||||||||
| Amortization | |||||||||||
| Research and development credits | |||||||||||
Capitalized research and development costs |
|||||||||||
| Lease liability | |||||||||||
| State taxes | |||||||||||
| Other | |||||||||||
| Gross deferred tax assets |
|
|
|||||||||
| Valuation allowance | ( |
( |
|||||||||
| Net deferred tax assets | $ |
|
$ |
|
|||||||
| Deferred tax liabilities | |||||||||||
| Right-of-use asset | $ | ( |
$ | ( |
|||||||
| Total deferred tax liabilities | ( |
( |
|||||||||
| Net deferred tax assets | $ |
|
$ |
|
|||||||
F-32
The provision for income taxes on earnings subject to income taxes differs from the statutory Federal rate at December 31, 2023 and 2022, due to the following:
| As of December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Expected income tax benefit at federal statutory tax rate | $ | ( |
$ | ( |
|||||||
| State income taxes, net of federal benefit | ( |
( |
|||||||||
| Change in fair value of warrants | ( |
||||||||||
| Change in valuation allowance | ( |
||||||||||
| Uncertain tax positions | |||||||||||
| Reduction in deferreds upon divestiture | |||||||||||
| Non-deductible interest | |||||||||||
| Stock compensation | |||||||||||
| Research and development credits | ( |
( |
|||||||||
| Rate adjustment | ( |
||||||||||
Foreign rate differential |
( |
||||||||||
Divestiture of VDL |
|||||||||||
In process research and development |
|||||||||||
Other |
( |
||||||||||
| Provision for income taxes | $ |
|
$ |
|
|||||||
The Company records a valuation allowance against deferred tax assets to the extent that it is more likely than not that some portion, or all of, the deferred tax assets will not be realized. Due to the the substantial doubt related to the Company's ability to utilize its deferred tax assets, a valuation allowance for the full amount of the deferred tax assets has been established at December 31, 2023. During the year ended December 31, 2023, the valuation allowance increased by $22,504,068 .
The Tax Cuts and Jobs Act of 2017 subjects a U.S. shareholder to tax on global intangible low-taxed income ("GILTI") earned by certain foreign subsidiaries. The FASB Staff Q&A, Topic 740, No. 5, Accounting for Global Intangible Low-Taxed Income, states that an entity can make an accounting policy election to recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense only. The Company elects to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense only.
Under the FASB’s accounting guidance related to income tax positions, among other things, the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. Additionally, the guidance provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
A reconciliation of the beginning and ending amounts of unrecognized tax positions are as follows:
| As of December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Unrecognized tax positions, beginning of the year | $ |
|
$ |
|
|||||||
| Gross increase - current period tax positions | |||||||||||
| Gross increase - prior period tax positions | |||||||||||
| Gross decrease – prior period tax positions | ( |
||||||||||
| Unrecognized tax positions, end of year | $ |
|
$ |
|
|||||||
If recognized, none of the unrecognized tax positions would impact the Company’s income tax benefit or effective tax rate as long as the Company’s net deferred tax assets remain subject to a full valuation allowance. The Company does not expect any significant increases or decreases to the Company’s unrecognized tax positions within the next twelve months.
F-33
11. Licensed Intellectual Property
UM 5050
The Company in-licenses the intellectual property used in its glaucoma product, SBI-100 OE, from the University of Mississippi under an "all fields of use" license. The license grants the Company an exclusive, perpetual license, including, with the prior written consent of UM, not to be unreasonably withheld, the right to sublicense.
The License Agreement provides for an annual maintenance fee of $75,000 payable on the anniversary of the effective date. The remaining milestone payments under the license are as follows:
i) $200,000 paid within 30 days following the first submission of an Investigational New Drug Application ("NDA") , or an equivalent application to a regulatory agency anywhere in the world, for each product that is administered in a different route of administration from that of the early submitted product(s); and
ii) $400,000 paid within 30 days following the approval of an NDA, or an equivalent application to a regulatory agency anywhere in the world, for each product that is administered in a different route of administration from that of the early approved product(s).
The royalty percentage due on net sales under each License Agreement is in the mid-single digits. The Company must also pay to UM a portion of all licensing fees received from any sublicensees, subject to a minimum royalty on net sales, and the Company is required to reimburse patent costs incurred by UM related to the licensed products. The royalty obligations apply by country and by licensed product, and end upon the later of the date that no valid claim of a licensed patent covers a licensed product in a given country, or ten years after the first commercial sale of such licensed product in such country.
Each License Agreement continues, unless terminated, until the later of the expiration of the last to expire of the patents or patent applications within the licensed technology or the expiration of the Company’s payment obligations under such License Agreement.
In July 2022, the Company paid $100,000 upon submitting its Investigational New Drug Application to the Food and Drug Administration for authorization to conduct the Company's Phase 1 trial of SBI-100 OE to the Therapeutic Goods Administration in Australia.
UM 5070 and 8930 License Agreements
Until January 8, 2022 and January 30, 2024, the Company licensed UM 5070 and UM 8930, respectively. Under these agreements, the Company was required to pay annual maintenance fees and certain milestones. However, after further evaluation, both licenses were terminated.
Tautomer Exclusive License Agreement
12. Related Party Matters
Emerald Health Sciences
In January 2018, the Company entered into a securities purchase agreement with Sciences pursuant to which Sciences purchased a majority of the equity interest in the Company, resulting in a change in control (the "Emerald Financing"). While Sciences no longer maintains a controlling interest in the Company, MFDI has significant influence over Sciences and has been issued the Convertible Note from the Company (Note 6) and participated in the August 2023 PIPE Financing (Note 7). As of December 31, 2023, the Amended Credit Agreement has been extinguished and all of the warrants held by Sciences were exercised pursuant to the MTA (Notes 5 & 6).
F-34
On May 18, 2022, Jim Heppell resigned from the Company's board of directors and concurrently entered into a consulting agreement with the Company pursuant to which Mr. Heppell will provide services mutually agreed upon by the Company. The consulting agreement has an initial minimum term of one-year and will be automatically renewed for a one-year period on the anniversary of the contract unless terminated with 60 days' notice. Under the consulting agreement, Mr. Heppell is entitled to a monthly fee of $6,300 , which was increased to $16,600 per month upon the closing of the EHT Acquisition. The consulting agreement provided Mr. Heppell with a termination payment of $74,700 on March 1, 2023, equal to the monthly fees through the then-remaining term of the agreement if Mr. Heppell’s engagement was terminated by the Company without cause. In addition, Mr. Heppell was awarded 16,000 stock options which are subject to certain performance and other conditions. On February 9, 2023, the Company provided notice and terminated the consulting agreement with Mr. Heppell effective March 11, 2023 and effective March 10, 2023, Mr. Heppell was removed from the Board of Sciences and no longer serves as Sciences CEO. During the year ended December 31, 2023, the first tranche of stock options issued to Mr. Heppell were cancelled, unexercised, and the second tranche of stock options were cancelled upon the closing of the Verdélite SPA.
The Company accounted for the consulting contract as an in-substance severance arrangement. During the year ended December 31, 2023, no severance expense was recognized. The Company recognized $139,615 in severance expense during the year ended December 31, 2022. The accrual for Mr. Heppell's severance was adjusted to include the increased fee payments when the Company closed the EHT Acquisition. As of December 31, 2022, the Company recognized $16,600 , in accounts payable - related party and $75,503 in other current liabilities - related party under this consulting agreement.
As of December 31, 2023, the Company no longer has any obligations or business relationship with Mr. Heppell.
VivaCell Biotechnology España, S.L.U (formerly known as Emerald Health Biotechnology España, S.L.U.)
In 2021, the Company entered into two separate Agreements pursuant to a Master Services Agreement with VivaCell Biotechnology España, S.L.U ("VivaCell"), a subsidiary of Emerald Health Research, Inc., which is 100 %-owned by Sciences. Under the Agreements, VivaCell will provide research and development services pursuant to agreed-upon project plans for the research and development of SBI-200 and the preclinical development services for novel derivatives. Payment for services are based on the negotiated amounts for the completion of agreed upon objectives as provided in the Agreements. The Company did not incur any expenses for the year ended December 31, 2023. For the year ended December 31, 2022, the Company incurred $87,927 in expenses under the Agreement.
In 2021, the Company entered into an Exclusive Sponsored Research Agreement (the “ESRA”) with VivaCell to fund certain research and development programs. The Company will have the right to use all data, products, and information, including intellectual property, which are generated in the performance of the research under each and all projects funded by the Company pursuant to the ESRA. VivaCell assigns and agrees to assign to the Company all rights to any intellectual property created or reduced-to-practice under or as a part of a project funded by the Company pursuant to the ESRA.
The Company has agreed to pay to VivaCell a royalty based on any and all licensing revenue or other consideration paid to the Company by a third-party licensee, assignee or purchaser of intellectual property rights created under the ESRA. For the years ended December 31, 2023 and 2022, the Company incurred $50,000 and $200,000 , respectively, in research and development expenses related to the retainer under the ESRA. As of December 31, 2023 and 2022, the Company has recognized $0 and $50,000 in accounts payable - related parties, respectively, related to the retainer under the ESRA.
On March 1, 2022, the Company entered into a research project with VivaCell under the ESRA Agreement for the development of a screening platform for anteroposterior ocular diseases. The project budget is $190,500 . For the years ended December 31, 2023 and 2022, the Company incurred $39,167 and $167,000 , respectively, of research and development expenses under the ESRA. As of December 31, 2023 and 2022, the Company recognized $0 and $7,835 in other current liabilities, and $0 and $47,001 in accounts payable- related parties under this agreement.
On May 8, 2023, the Company terminated the ESRA effective March 31, 2023, and Vivacell waived the required notice period under the ESRA.
Management Conflicts
Until the date of the EHT Acquisition, the Company's CEO, Punit Dhillon, was a board member of the Company and EHT (Note 3).
On February 28, 2022, the Company entered into a standard consulting agreement with the CEO's brother to assist with diligence on the EHT Acquisition due to his knowledge and expertise as a former executive of EHT. Compensation under the agreement is for a rate of approximately $73 per hour. The consulting agreement may be terminated by either party upon providing 15 days of advance notice. For the years ended December 31, 2023 and 2022, the Company incurred $35,087 and $46,684 , respectively, in consulting expenses under this agreement. As of December 31, 2023 and 2022, the Company recorded $0 and $12,511 to other current liabilities - related parties related to this consulting agreement. Effective June 30, 2023, this contract was terminated.
F-35
13. Commitments and Contingencies
Office Lease
The Company leases office space for its corporate headquarters, located at 11250 El Camino Real, Suite 100 San Diego, California 92130. The original lease term was effective from September 1, 2021 through October 31, 2023 and contained a renewal option for a two-year extension after the current expiration date. At the commencement date, the Company did not expect to exercise the renewal option, and has therefore excluded the option from the calculation of the right of use asset and lease liability. The lease provides for two months of rent abatement and the initial monthly rent is $8,067 per month with annual increases of 3 % commencing on November 1, 2022. The lease included non-lease components (i.e., property management costs) that are paid separately from rent, based on actual costs incurred, and therefore were not included in the right-of-use asset and lease liability but are reflected as an expense in the period incurred. In calculating the present value of the lease payments, the Company has elected to utilize its incremental borrowing rate based on the lease term.
The Company entered into an amended and restated lease agreement on June 27, 2023 for its corporate headquarters, extending the lease term to 36 months, retroactive to September 1, 2021 through October 31, 2026. The Company treated the amended and restated lease agreement as a single modified lease.
For the years ended December 31, 2023 and 2022, lease expense comprised of $97,986 and $90,701 , respectively in lease cost from the Company's non-cancellable operating lease.
The remaining lease term and discount rate related to the operating lease are presented in the following table:
| December 31, 2023 | |||||
| Weighted-average remaining term – operating lease (in years) | |||||
| Weighted-average discount rate – operating lease | % | ||||
Future minimum lease payments as of December 31, 2023 are presented in the following table:
| Year: | |||||
| 2024 | |||||
| 2025 | |||||
| 2026 | |||||
| Total future minimum lease payments: | |||||
| Less imputed interest | ( |
||||
| Total | $ |
|
|||
Reported as:
| December 31, 2023 | December 31, 2022 | ||||||||||
| Operating lease liability | $ | $ | |||||||||
| Operating lease liability, net of current portion | |||||||||||
| Total lease liability | $ |
|
$ |
|
|||||||
General Litigation and Disputes
From time to time, in the normal course of operations, the Company may be a party to litigation and other dispute matters and claims. Litigation can be expensive and disruptive to normal business operations. Moreover, the results of complex legal proceedings are difficult to predict. An unfavorable outcome to any legal matter, if material, could have a materially adverse effect on the Company’s operations or financial position, liquidity or results of operations.
F-36
Wendy Cunning vs Skye Bioscience, Inc.
The Company is a party to a legal proceeding with a former employee alleging, among other things, wrongful termination, violation of whistleblower protections under the Sarbanes-Oxley Act of 2002, and retaliation under California law against the Company relating to certain actions and events that occurred with the Company's former management during the employee's employment term from March 2018 to July 2019. The case, entitled Wendy Cunning vs Skye Bioscience, Inc., was filed in U.S. District Court (the "District Court") for the Central District of California (the “Cunning Lawsuit”). On January 18, 2023, a jury rendered a verdict in favor of Ms. Cunning and awarded her $512,500 in economic damages (e.g., lost earnings, future earnings and interest), $840,960 in non-economic damages (e.g., emotional distress) and $3,500,000 in punitive damages. On February 13, 2023, the Company received the final judgment on the special verdict (the "Final Judgment") from the District Court.
On August 2, 2023, the District Court ruled on the plaintiff's motion for attorney fees and awarded the plaintiff $1,200,008 . Based on this order, the Company reduced the aggregate estimate for the legal contingency by $151,842 , the difference between the attorney fees awarded by the District Court and the Company's previous estimate.
Immediately prior to the closing of the PIPE Financing, on August 17, 2023, the Company obtained a stay on enforcement of the judgment in the Cunning Lawsuit by posting an appeal bond in the amount of $9,080,202 .
On October 19, 2023, the Company received the final orders from the District Court denying the post-trial motions that the Company filed with the District Court in March 2023 seeking judgment as a matter of law, a new trial, and/or a reduction of the judgment. Additionally, in March of 2023, the Company appealed the judgment in the Cunning Lawsuit with the Ninth District Court of Appeals, which is moving forward now that the District Court has ruled on the post-trial motions. In March 2024, the Company filed the opening brief for the appeal with the Ninth District Court of Appeals.
The Company strongly believes that this case was incorrectly decided as to liability, the amount of compensatory damages, and the appropriateness and amount of punitive damages. The Company is challenging the verdict in the Ninth District Court of Appeals and is pursuing reimbursement under its existing insurance policies, but given the jury verdict, the Company has determined that a loss is probable and accordingly have recorded a legal contingency expense and a current balance sheet liability for the total amount of the jury verdict. The Company has recorded an aggregate estimate for the legal contingency of $6,053,468 plus accrued interest of $234,750 at an annual interest rate of 4.87 % on the judgment and 5.38 % on the legal fees, which is determined by the Superior Court of California. Depending on the judge's final order on the post-trial motions and appeal, it is reasonably possible that the legal contingency booked could materially change after the issuance of these financials.
Skye Bioscience, Inc. vs Partner Re Ireland Insurance
In February 2023, the Company brought a suit against the Company's D&O carrier, Partner Re Ireland Insurance DAC ("Partner Re"), bringing claims for (a) breach of contract, (2) tortious breach of the implied covenant of good faith and fair dealing and (3) declaratory relief that Partner Re is obligated to reimburse the Company for the defense fees and costs incurred in defense of the Cunning Lawsuit and must indemnify the Company for any settlement or judgment in the Cunning Lawsuit. The Company's allegations arise out of Partner Re's refusal to reimburse the Company for costs incurred by the Company in defending the Cunning Lawsuit. The case, entitled Skye Bioscience, Inc., v. Partner Re Ireland Insurance DAC, was filed in the United Stated District Court for the Central District of California.
On April 17, 2023, Partner Re filed a motion to dismiss the Company's complaint pursuant to Federal Rule of Civil Procedure 12(b)(6). On June 20, 2023, the judge issued a final ruling in favor of the Company and denied Partner Re's motion to dismiss the Company's lawsuit. In its ruling, the Court rejected Partner Re's primary basis for denying coverage.
Based on the outcome, the Company is pursuing up to $5,000,000 in coverage less the deductible to cover legal expenses incurred and the final verdict or settlement of the Cunning Lawsuit.
14. Subsequent Events
Sale of Real Estate Held by AVI
On January 15, 2024, the Company closed the sale of the real estate held by AVI, which comprised of substantially all of AVI's assets.. The real estate and related equipment was sold to Tab Labs, Inc. for an aggregate purchase price of $1,139,572 .
F-37
PIPE Financings
January 2024 PIPE Financing
On January 29, 2024, the Company entered into a Securities Purchase Agreement, pursuant to which on January 31, 2023, the Company issued an aggregate of 11,713,664 shares of common stock and 9,978,739 pre-fund warrants to purchase up to 9,978,739 shares of common stock (the "January 2024 PIPE Financing") for an aggregate purchase price of $49,991,010 . The January 2024 PIPE Financing was priced at $2.31 per common share and per $2.30 pre-funded warrant based on the 5-day average share price preceding January 29, 2024. The pre-funded warrants are exercisable at any time for $0.001 .
In connection with the PIPE Financing, the Company incurred $3,824,841 in direct equity issuance costs for net proceeds of $46,166,169 .
March 2024 PIPE Financing
On March 11, 2024, the Company entered into a Securities Purchase Agreement, pursuant to which on March 13, 2024, the Company issued an aggregate of 4,000,000 shares of common stock (the "March 2024 PIPE Financing") for an aggregate purchase price of $40,000,000 . The March 2024 PIPE Financing was priced at $10.00 per common share.
In connection with the PIPE Financing, the Company incurred $2,625,000 in direct equity issuance costs for net proceeds of approximately $37,375,000 .
Stock-based Compensation
On February 29, 2024, the Company granted certain employees and directors 275,000 RSUs with market based vesting conditions. The RSUs vest on the following milestones: (i) 25 % vests upon achieving a market cap of $750,000,000 and a stock price of $20.00 per share, (ii) 25 % vests upon achieving a market cap of $1,000,000,000 and a stock price of $30.00 per share, (iii) 25 % vests upon achieving a market cap of $1,250,000,000 and a stock price of $32.50 per share, and (iv) 25 % vest upon achieving a market cap of $1,500,000,000 or greater and a stock price of $35.00 per share; provided, however, that no RSUs shall vest until the compensation committee of the Company determines that shares can be sold into the market to cover withholding tax obligations associated with the vesting of the RSUs. Upon a change in control of the Issuer, 100 % of the RSUs will become fully vested.
F-38
The following exhibits are filed with this Annual Report on Form 10-K.
Exhibit Number
|
Description of Exhibit | |||||||
| 2.1 | ||||||||
| 2.2 | ||||||||
| 2.3 | ||||||||
| 2.4 | ||||||||
| 2.5 | ||||||||
| 2.6 | ||||||||
| 2.7 | ||||||||
| 2.8 | ||||||||
2.9* |
||||||||
3.1* |
||||||||
| 3.2 | ||||||||
| 4.1 | ||||||||
| 4.2 | ||||||||
| 4.3 | ||||||||
| 4.4 | ||||||||
| 4.5 | ||||||||
| 4.6 | ||||||||
| 4.7 | ||||||||
| 4.8 | ||||||||
| 4.9 | ||||||||
4.10 |
||||||||
4.11 |
||||||||
60
4.12 |
||||||||
4.13 |
||||||||
4.14* |
||||||||
| 10.1† | ||||||||
| 10.2† | ||||||||
10.3† |
||||||||
10.4† |
||||||||
10.5† |
||||||||
10.6† |
||||||||
10.7† |
||||||||
10.8† |
||||||||
10.9† |
||||||||
10.10† |
||||||||
10.11† |
||||||||
| 10.12** | ||||||||
| 10.13 | ||||||||
10.14** |
||||||||
10.15** |
||||||||
| 10.16 | ||||||||
10.17** |
||||||||
10.18** |
||||||||
10.19** |
||||||||
61
| 10.2 | ||||||||
| 10.21 | ||||||||
| 10.23 | ||||||||
| 21.1* | ||||||||
23.1* |
||||||||
| 31.1* | ||||||||
| 31.2* | ||||||||
32.1* |
||||||||
32.2* |
||||||||
97.1*† |
||||||||
101* |
Inline XBRL Document Set for the consolidated financial statements and accompanying notes in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K |
|||||||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |||||||
_________
* Filed Herewith
** Portions of this exhibit have been omitted in compliance with Regulation S-K Item 601(b)(10)(iv).
† Management contract or compensatory plan or arrangement.
Item 16. Form 10-K Summary.
None.
62
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Skye Bioscience, Inc.
a Nevada corporation
|
|||||||||||
| March 21, 2024 | By: | /s/ Punit Dhillon | |||||||||
| Punit Dhillon | |||||||||||
| Its: | Chief Executive Officer, Chairman | ||||||||||
| (Principal Executive Officer) | |||||||||||
| March 21, 2024 | By: | /s/ Kaitlyn Arsenault | |||||||||
| Kaitlyn Arsenault | |||||||||||
| Its: | Chief Financial Officer | ||||||||||
| (Principal Financial and Accounting Officer) | |||||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
63
| By: | /s/ Punit Dhillon | March 21, 2024 | |||||||||
| Punit Dhillon | |||||||||||
| Its: | Chief Executive Officer, Chairman | ||||||||||
| (Principal Executive Officer) | |||||||||||
| By: | /s/ Kaitlyn Arsenault | March 21, 2024 | |||||||||
| Kaitlyn Arsenault | |||||||||||
| Its: | Chief Financial Officer | ||||||||||
| (Principal Financial and Accounting Officer) | |||||||||||
| By: | /s/ Margaret Dalesandro | March 21, 2024 | |||||||||
| Margaret Dalesandro | |||||||||||
| Its: | Director | ||||||||||
| By: | /s/ Deborah Charych | March 21, 2024 | |||||||||
| Deborah Charych | |||||||||||
| Its: | Director | ||||||||||
| By: | /s/ Praveen Tyle | March 21, 2024 | |||||||||
| Praveen Tyle | |||||||||||
| Its: | Director | ||||||||||
| By: | /s/ Keith Ward | March 21, 2024 | |||||||||
| Keith Ward | |||||||||||
| Its: | Director | ||||||||||
| By: | /s/ Andrew Schwab |
March 21, 2024 | |||||||||
Andrew Schwab |
|||||||||||
| Its: | Director | ||||||||||
| By: | /s/ Paul Grayson |
March 21, 2024 | |||||||||
Paul Grayson |
|||||||||||
| Its: | Director | ||||||||||
| By: | /s/ Annalisa Jenkins |
March 21, 2024 | |||||||||
Annalisa Jenkins |
|||||||||||
| Its: | Director | ||||||||||
64